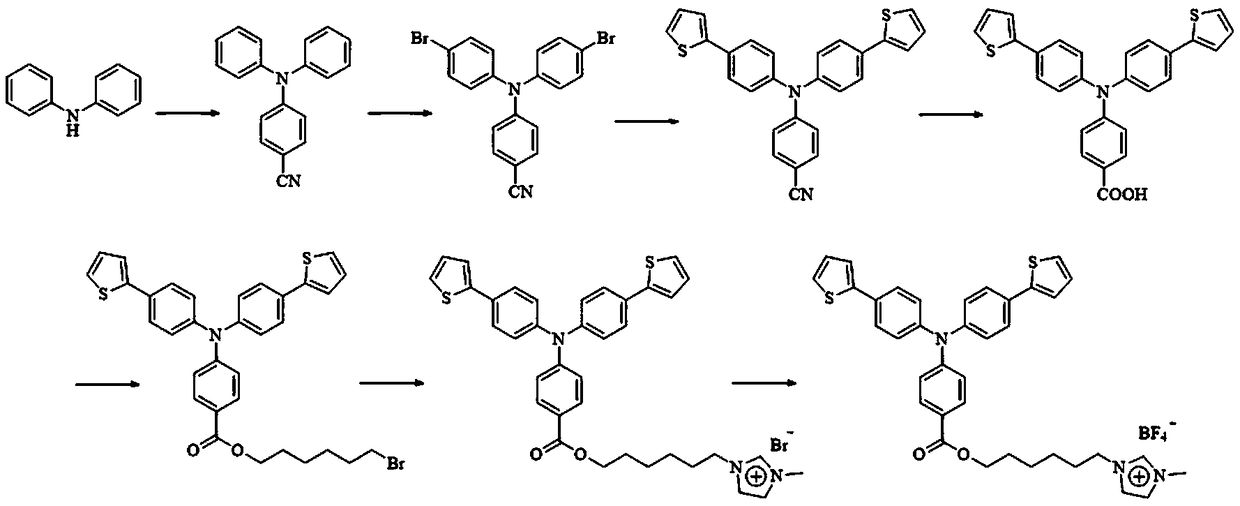
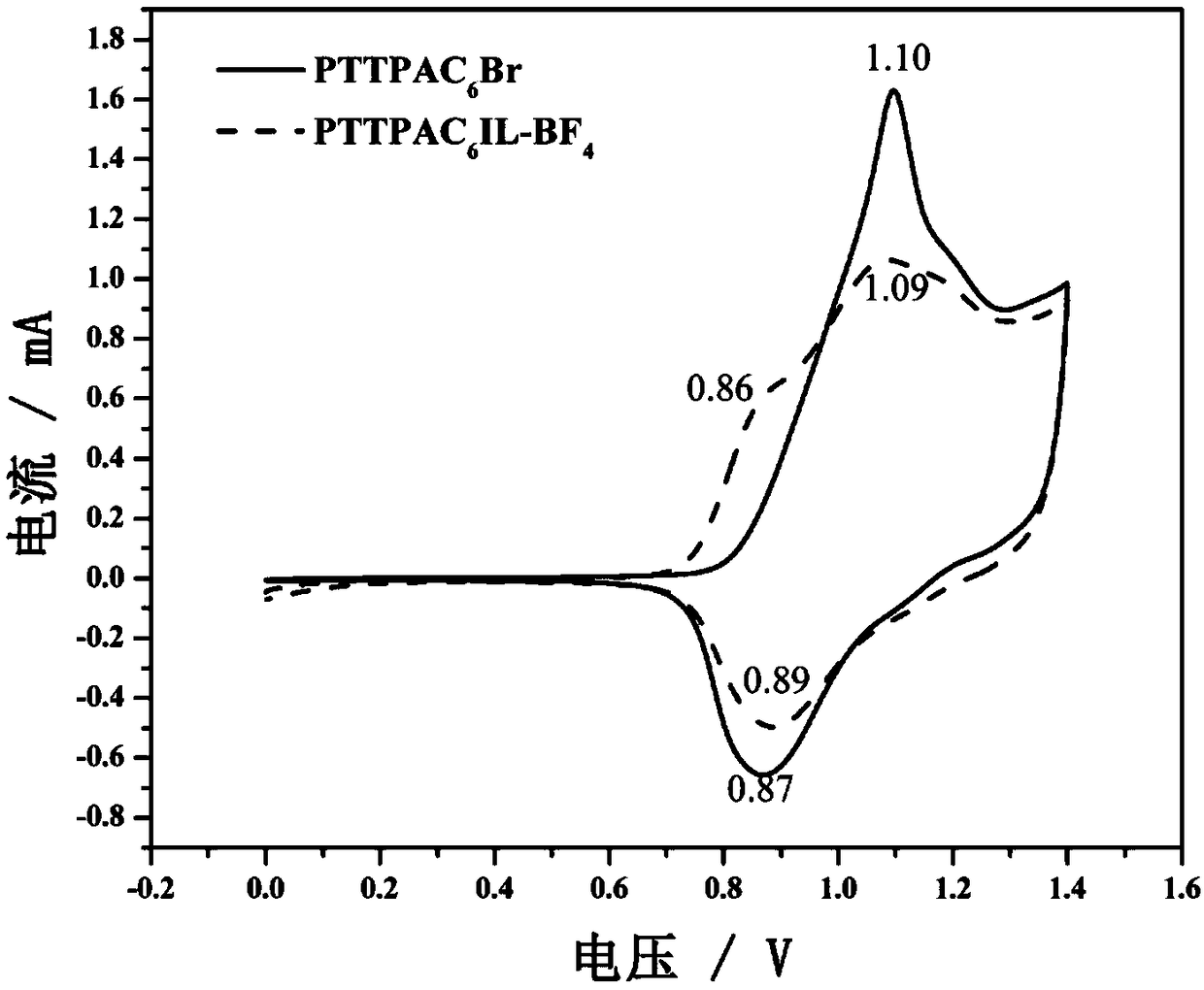
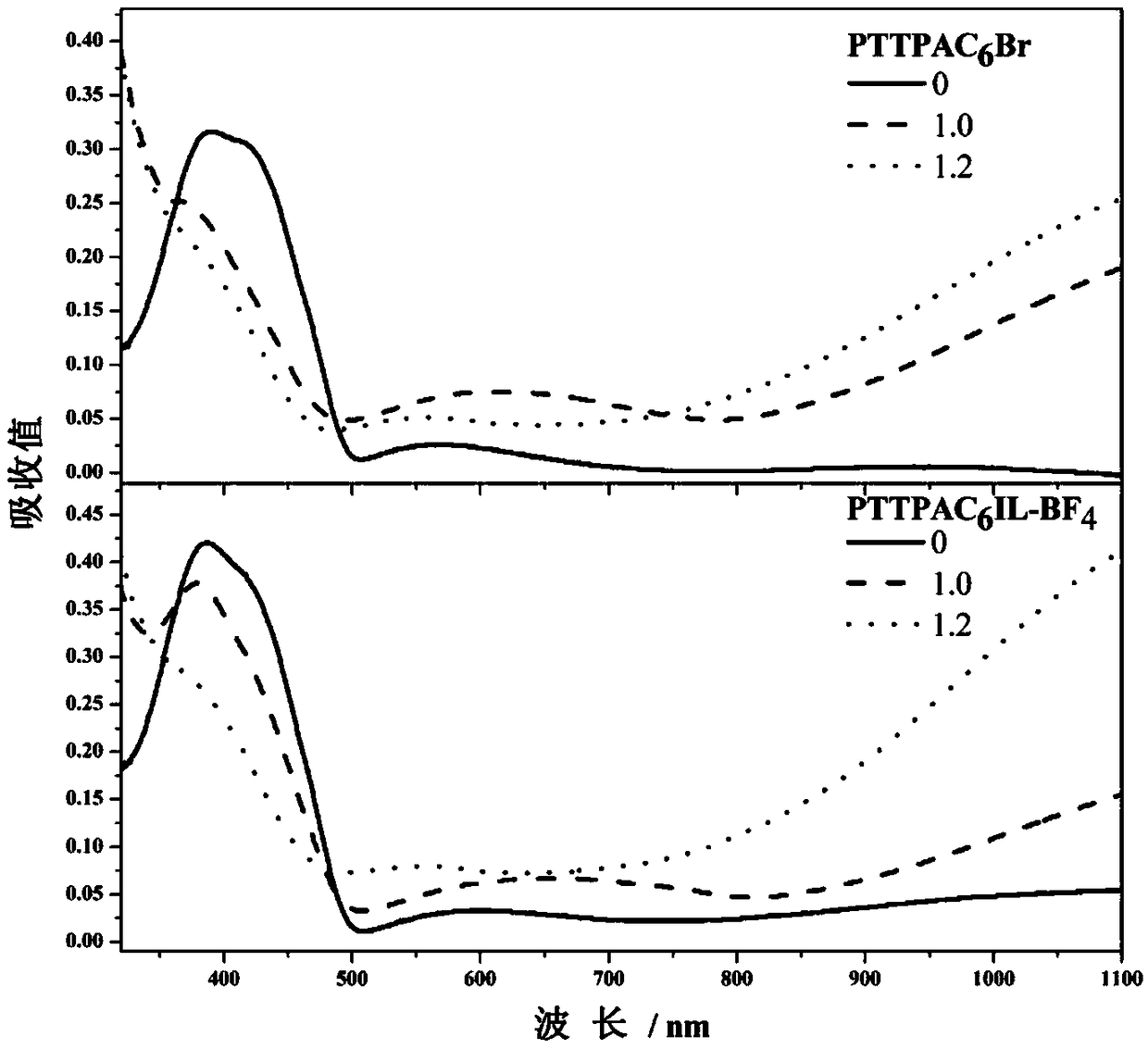
Material of imidazole type ionic liquid modified triphenylamine derivative and preparation method and application thereof
A technology of derivatives and triphenylamines, which is applied to materials based on imidazole-type ionic liquid-modified triphenylamine derivatives and the fields of preparation and application thereof, can solve the problems of high cost and expensive ionic liquids, and achieve fast response time, The effect of high optical contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 4-(diphenylamino) benzonitrile
[0038] Under nitrogen protection, sodium hydride (1.496 g, 62.3 mmol) was dissolved in DMF (40 mL), and stirred well until no bubbles were generated. Diphenylamine (5.11g, 30.2mmol) was added to the solution, and after the temperature rose to 110°C and stabilized, p-fluorobenzonitrile (4.49g, 37.1mmol) was slowly added, and the system was refluxed for 12h. After the reaction was completed, the reaction liquid was cooled to room temperature, and saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Use 300-400 mesh silica gel as the stationary phase, elute with dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase, collect the eluate containing the target compound, distill off the solvent under re...
Embodiment 2
[0039] Example 2 Synthesis of 4-(bis(4-bromophenyl)amino)benzonitrile
[0040] Under dark conditions, the intermediate product 4 (dianilino) benzonitrile compound (4 g, 14.8 mmol) was dissolved in DMF (30 mL), and NBS (6.59 g, 37.1 g) dissolved in DMF (15 mL) was added dropwise. mmol) solution, reacted at room temperature for 24h. After the reaction was completed, the reaction liquid was cooled to room temperature, and saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Use 300-400 mesh silica gel as the stationary phase, elute with dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase, collect the eluate containing the target compound, distill off the solvent under reduced pressure and dry to obtain a white The powdery intermediate product 4(bis(4-bromophenyl)a...
Embodiment 3
[0041] Example 3 Synthesis of 4-(bis(4-(thiophene-2)phenyl)amino)benzonitrile
[0042] Under nitrogen protection, the intermediate product 4-(bis(4-bromophenyl)amino)benzonitrile (3.75g, 8.76mmol), potassium carbonate (5.4g, 39.12mmol), bis(triphenylphosphine) di Palladium chloride (780mg, 1.11mmol) and 2-thiopheneboronic acid (3.9g, 30.47mmol) were dissolved in a mixed solution of methanol (50mL) and toluene (50mL), and reacted at 100°C for 24h. After the reaction was completed, the reaction liquid was cooled to room temperature, and saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Use 300-400 mesh silica gel as the stationary phase, elute with dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase, collect the eluate containing the target compound, distill of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com