Application of polypeptide capable of passing through hemato encephalic barrier in preparing drug
A technology for preparing medicines and medicines, which is applied in the application field of fusion peptides in the preparation of medicines, and can solve the problems that the analgesic effect and shelf life cannot reach clinical application, cannot carry out good quality control, and cannot carry out large-scale promotion, etc. , to achieve the effect of long drug effect, excellent analgesic effect and low clinical risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 different types of ziconotide fusion peptides
[0043] Four different types of fusion peptides were prepared and named as protected polypeptides MVIIA-a, MVIIA-b, MVIIA-c, and MVIIA-d, respectively. At the same time, ziconotide was prepared and named MVIIA as a control. In this experiment, the F-moc automatic solid-phase synthesis method was adopted, and the specific steps are as follows:
[0044] Synthesis of polypeptides: Protected polypeptides and their derivatives were assembled on resin using a model of the 433A automatic synthesizer (ABI, Foster City, CA). The peptide resin was incubated in suspension for 2.5 hours at room temperature to deprotect the group. The suspension system is composed of 10 ml of TFA, 0.75 g of phenol, 0.25 ml of 1,2-ethanedithiol, 0.5 ml of sulfide anisole and 0.5 ml of water. (Wat methoxycarbonyl (Fmoc), a common alkoxycarbonyl amino protecting group). The resin was separated from the peptide deprotecte...
Embodiment 2
[0047] Example 2: Chemical properties and structural characterization of different types of ziconotide fusion peptides
[0048] 1. Chemical properties of MVIIA and its variants
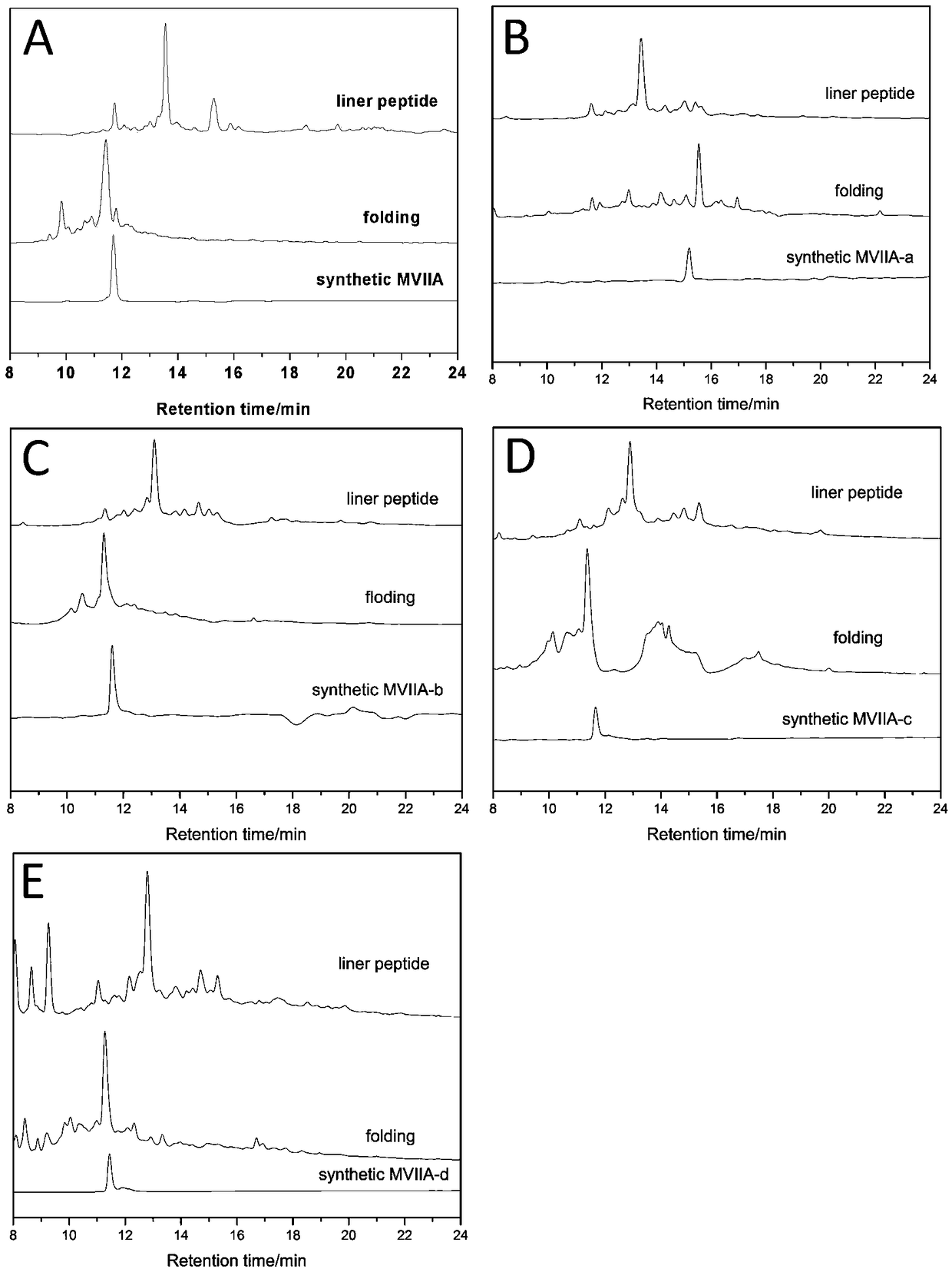
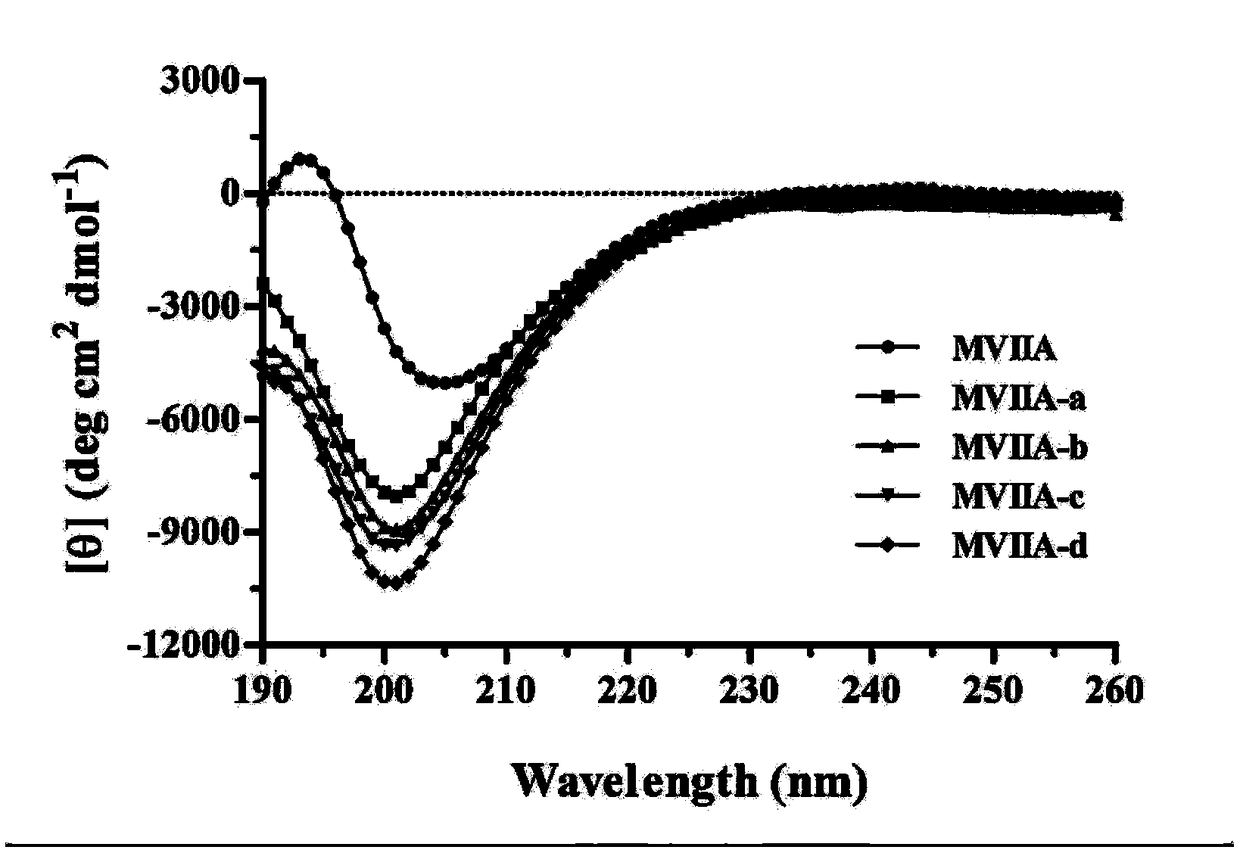
[0049] The buffer-treated linear peptides were treated for 24-48 hours at 4°C, and then analyzed by high-performance liquid chromatography. It was found that the folding of the linear peptides resulted in the appearance of a major peak and several minor peaks. The buffer system includes 1mM glutathione, 0.1mM oxidized glutathione, 1mM EDTA, and 0.2mg / mL linear peptide, and the pH of the solution is 7.9. The major product was purified and evaluated by analytical reversed-phase high-performance liquid chromatography, and the purity of the peptide was determined to be greater than 98%. Determination was performed with an Ultraflex III TOF / TOF mass spectrometer (Bruker). The prepared polypeptide sequence is shown in Table 1, and its one-step oxidative folding HPLC analysis profile is shown in figure 1 ...
Embodiment 3
[0058] Embodiment 3: Electrophysiological experiments of different types of ziconotide fusion peptides
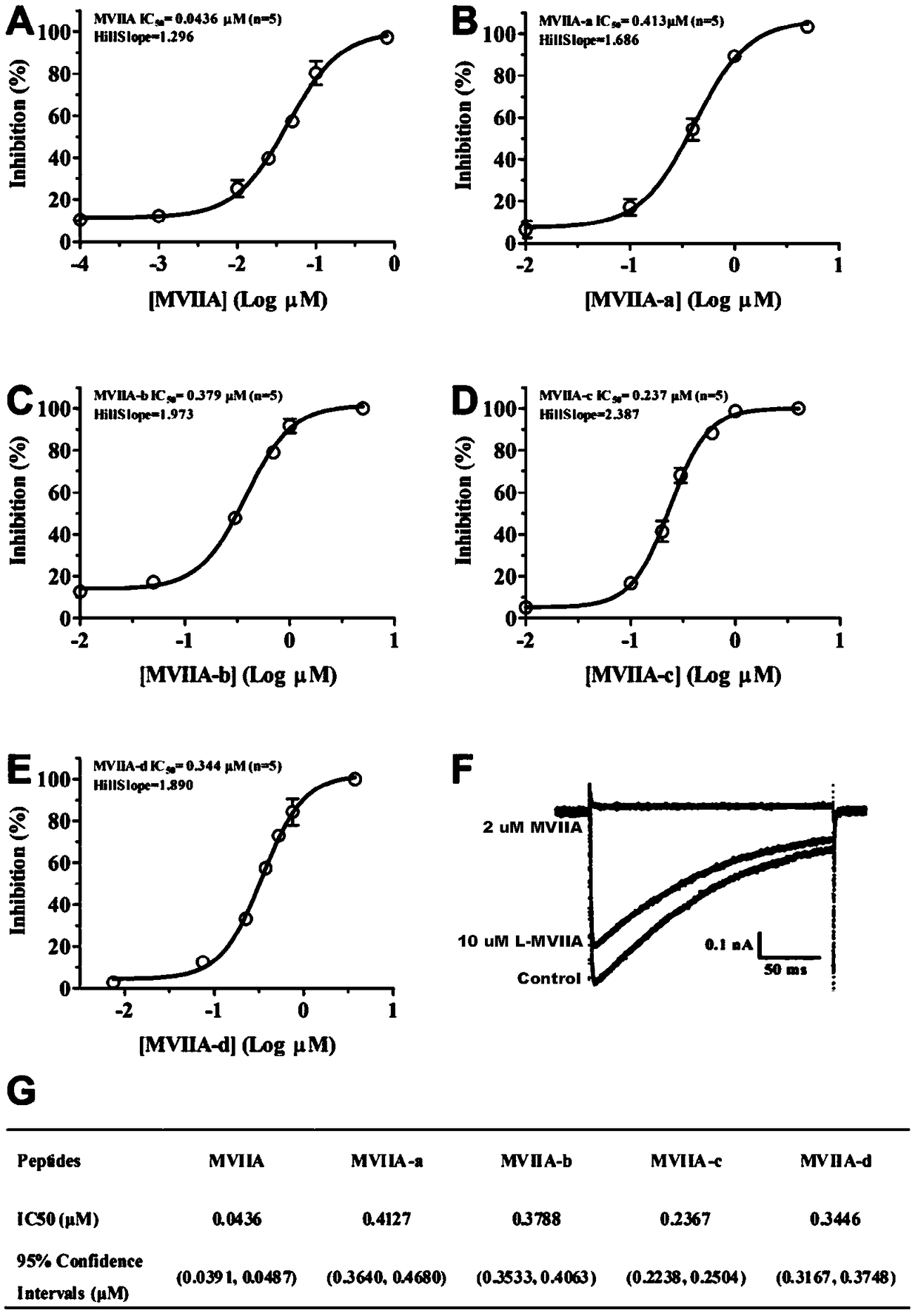
[0059] In order to further study the electrophysiological effects of different types of improved ziconotide and the inhibitory effect on calcium ion (CaV2.2) channels, the following experiments were carried out:
[0060] HEK293T cells (capable of expressing SV40 large T antigen) were cultured in DMEM high-glucose medium (Gibco) containing 10% fetal bovine serum, 1% penicillin and streptomycin. The incubator environment is 37°C, 5% CO 2 . Dr. Diane Lipscombe provided the alpha of the rat CaV2.2 channel 1B Splice variant e37a, auxiliary subunit α 2 δ 1 and beta 3 Plasmids (Addgene plasmid #26569, #26575, #26574). Then three kinds of plasmids (3μg), 0.4μg enhanced green fluorescent protein gene and liposome were transiently transfected into HEK293T cells. 24 hours after transfection, the cells were seeded on glass slides and incubated in an incubator (37°C, 5% CO 2 ) f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com