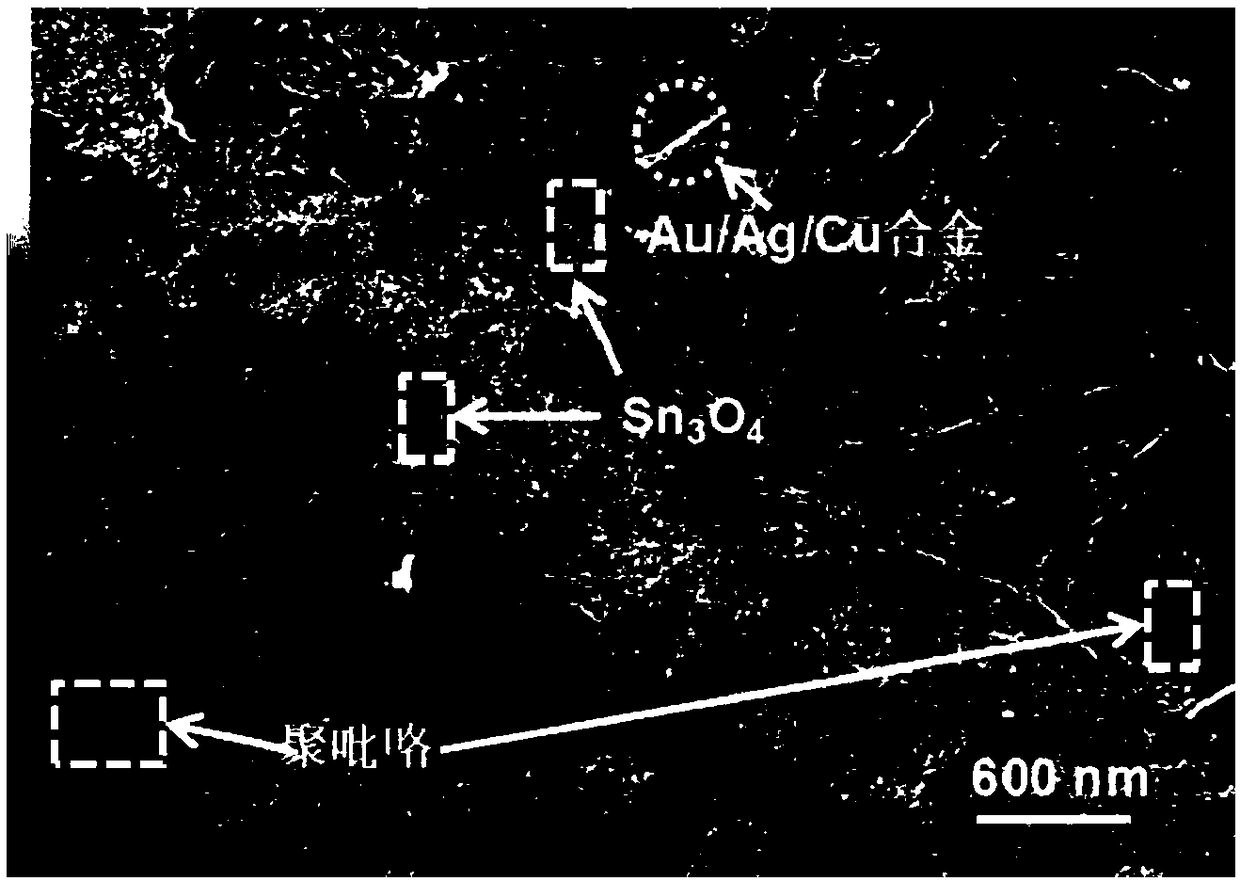
Preparation method of polypyrrole/metal-modified Sn3O4 nano composite photocatalytic material
A photocatalytic material and metal modification technology, applied in the field of preparation of nanocomposite photocatalytic materials, can solve the problems of obvious hard agglomeration effect of samples, easy falling off of supported metals, complicated preparation process, etc., and achieve excellent photocatalytic performance and particle size distribution. Narrow, low preparation temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) get 1mmol analytically pure stannous octoate (C 16 h 30 o 4 Sn) and 0.6mmol of acetic acid (CH 3 COOH), fully dissolved in 2mL of absolute ethanol, and then sequentially added 4mmol of sodium sorbate, 0.5mmol of phytic acid and 10mL of deionized water, the whole process was in an ice-salt bath of sodium chloride and crushed ice at -20°C Continue to use constant temperature magnetic stirring to obtain solution A evenly;
[0031] 2) get 0.05mmol analytically pure chloroplatinic acid (H 2 PtCl 6 ), 1mmol of silver nitrate (AgNO 3 ), 10mmol of copper nitrate (Cu(NO 3 ) 2 ) and 0.1 mmol of acetic acid (CH 3 COOH) was fully dissolved in 7mL of deionized water, and the whole process was continuously stirred with constant temperature magnetic force in an ice-salt bath of sodium chloride and crushed ice at -20°C to obtain solution B;
[0032] 3) Add solution B dropwise to solution A at a rate of 30 drops / minute, and continuously use constant temperature magnetic stir...
Embodiment 2
[0037] 1) get 1mmol analytically pure stannous octoate (C 16 h 30 o 4 Sn) and 2 mmol of acetic acid (CH 3 COOH), fully dissolved in 11mL of absolute ethanol, then sequentially added 10mmol of sodium sorbate, 3mmol of phytic acid and 15mL of deionized water, the whole process was in an ice salt bath of sodium chloride and crushed ice at -10°C Continuously use constant temperature magnetic stirring to obtain solution A evenly;
[0038] 2) Take 0.5mmol of chloroauric acid (HAuCl 4 ), 1mmol of silver nitrate (AgNO 3 ), 5mmol of copper nitrate (Cu(NO 3 ) 2 ) and 3mmol of acetic acid (CH 3 COOH) was fully dissolved in 10mL of deionized water, and the whole process was continuously stirred with constant temperature magnetic force in an ice-salt bath of sodium chloride and crushed ice at -10°C to obtain solution B;
[0039] 3) Add solution B dropwise to solution A at a rate of 40 drops / min, and continuously use constant temperature magnetic stirring in the ice-salt bath of sod...
Embodiment 3
[0045] 1) get 1mmol analytically pure stannous octoate (C 16 h 30 o 4 Sn) and 4.7mmol of acetic acid (CH 3 COOH), fully dissolved in 17mL of absolute ethanol, then sequentially added 18mmol of sodium sorbate, 6mmol of phytic acid and 26mL of deionized water, the whole process was continued in an ice-salt bath of sodium chloride and crushed ice at 0°C Use constant temperature magnetic stirring to obtain solution A evenly;
[0046] 2) Take 1mmol of chloroauric acid (HAuCl 4 ), 3mmol of silver nitrate (AgNO 3 ) and 6mmol of acetic acid (CH 3 COOH) was fully dissolved in 15mL of deionized water, and the whole process was continuously stirred with constant temperature magnetic force in an ice-salt bath of sodium chloride and crushed ice at 0°C to obtain solution B;
[0047] 3) Add solution B dropwise to solution A at a rate of 60 drops / minute, and continuously use constant temperature magnetic stirring in the ice-salt bath of sodium chloride and crushed ice at 0°C to obtain m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com