Preparation method of alpha-(2,4-dichlorophenyl)-1h-imidazole-1-ethanol
A technology of imidazole ethanol and ethanol, which is applied in the field of preparation of imidazole ethanol, can solve the problems of various reagents, affect the yield, increase the synthesis cost, etc., and achieve the effects of avoiding post-processing difficulties, simple and convenient operation, and favorable recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
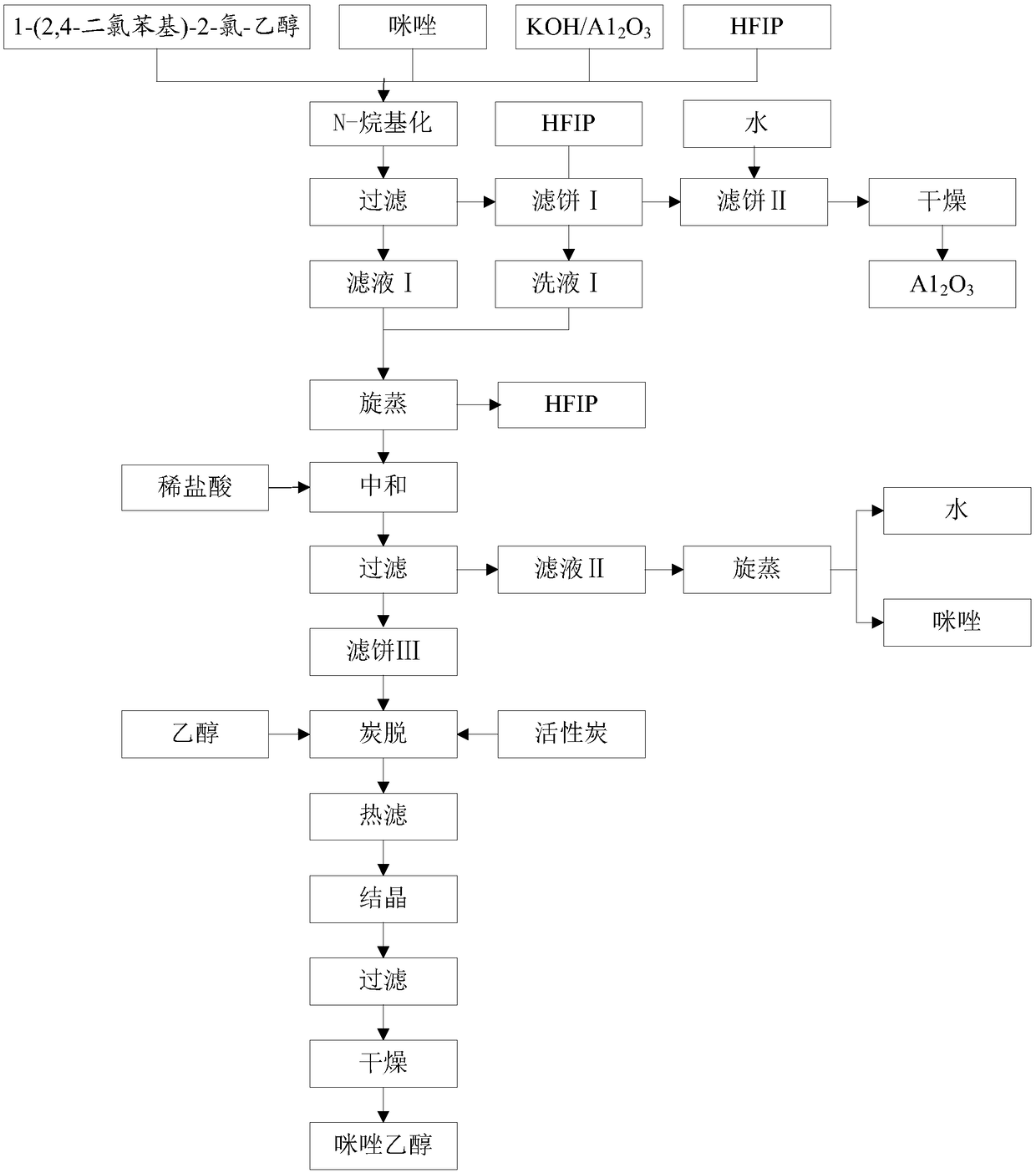
[0031] Such as figure 1 Shown flow chart, preparation imidazole ethanol, concrete steps are as follows:
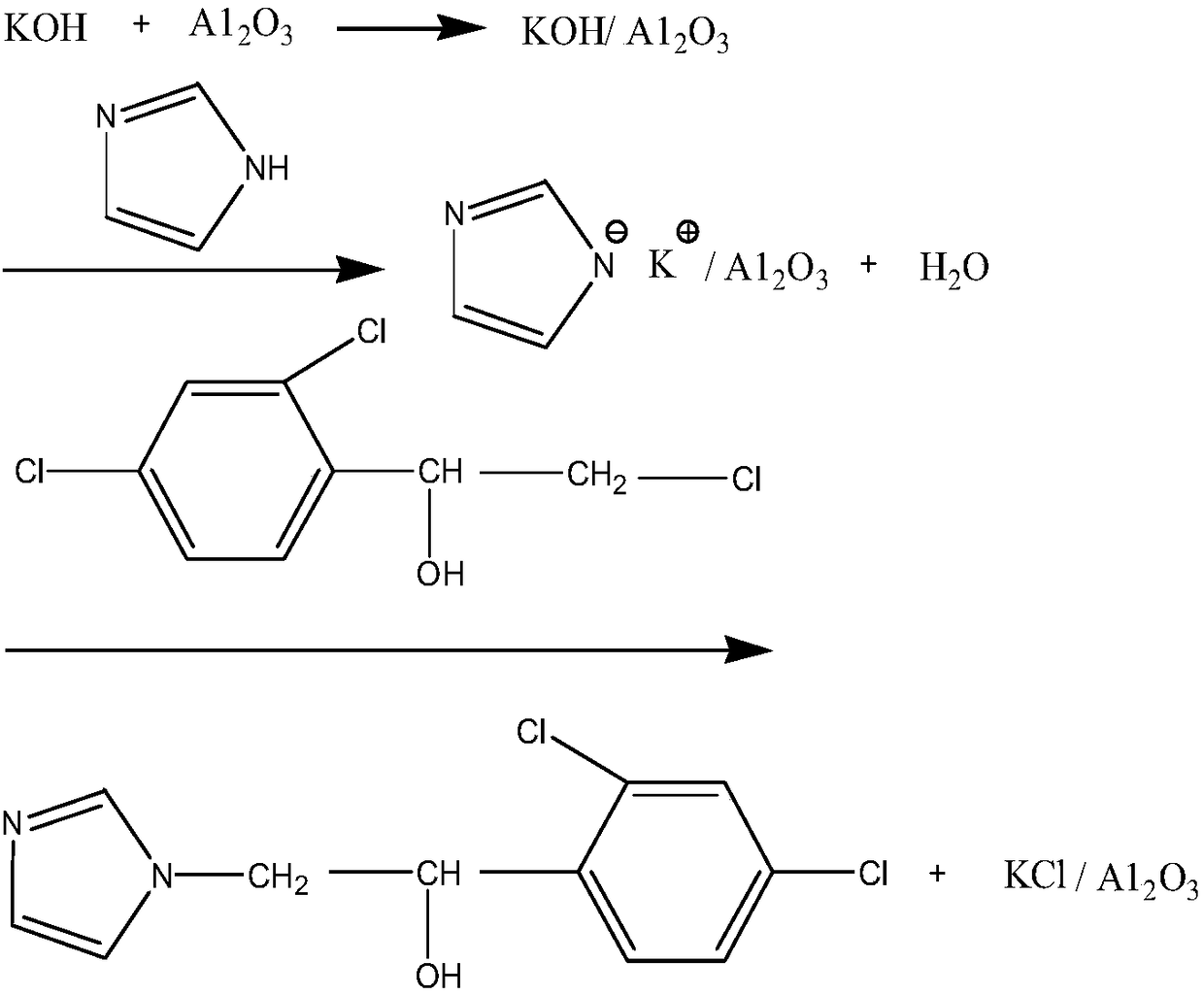
[0032] (1) KOH / A1 2 o 3 Preparation: Dissolve 15g of KOH in 20g of water, add A1 2 o 3 30g, stir and heat up to 60°C and keep it warm for 1h, rotary evaporation speed 70r / min, vacuum degree -0.09~-0.1MPa, control water temperature 80~90°C to distill the water to dryness, dry at 120°C for 4h to get KOH / A1 2 o 3 42.5g, placed in a desiccator for subsequent use;
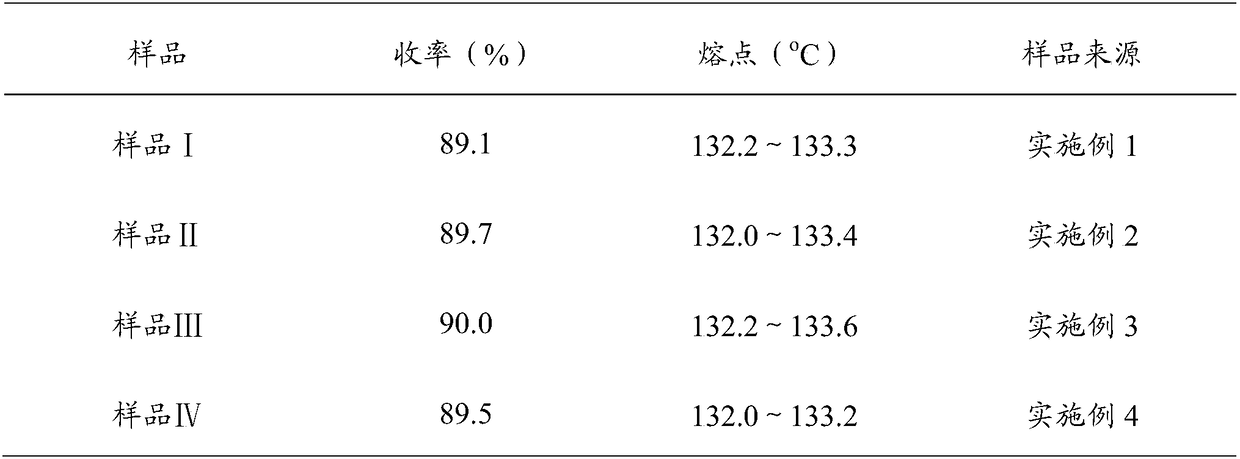
[0033] (2) Preparation of imidazole ethanol: add HFIP260g, KOH / A1 2 o 338g, 18.4g of imidazole, 44.7g of 1-(2,4-dichlorophenyl)-2-chloro-ethanol, heated to 45°C for 3.5h, followed by TLC, and filtered to obtain filter cake I and filtrate I, The filter cake I was rinsed with HFIP to obtain lotion I and filter cake II, combined the filtrate 1 and lotion 1, and carried out rotary evaporation, the control speed was 70r / min, the vacuum degree was -0.09~-0.1MPa, the water temperature was 40~50°C to no The disti...
Embodiment 2
[0036] (1) KOH / A1 2 o 3 Preparation: Dissolve 15g of KOH in 20g of water, add A1 2 o 3 30g, stir and heat up to 60°C and keep it warm for 1h, rotary evaporation speed 70r / min, vacuum degree -0.09~-0.1MPa, control water temperature 80~90°C to distill the water to dryness, dry at 120°C for 4h to get KOH / A1 2 0 3 41.7g, placed in a desiccator for subsequent use;
[0037] (2) Preparation of imidazole ethanol: Add HFIP 280g, KOH / A1 2 o 3 40g, 18.7g of imidazole, 44.7g of 1-(2,4-dichlorophenyl)-2-chloro-ethanol, heated to 45°C, reacted for 4h, tracked the reaction by TLC, filtered to obtain filter cake I and filtrate I, The filter cake I was rinsed with HFIP to obtain lotion I and filter cake II, combined the filtrate 1 and lotion 1, and carried out rotary evaporation, the control speed was 70r / min, the vacuum degree was -0.09~-0.1MPa, the water temperature was 40~50°C to no The distillate flows out, add 13g of 10% dilute hydrochloric acid (3.6g of 36% hydrochloric acid mi...
Embodiment 3
[0040] (1) KOH / A1 2 o 3 Preparation: Dissolve 15g of KOH in 20g of water, add A1 2 o 3 30g, stir and heat up to 60°C and keep it warm for 1h, rotary evaporation speed 70r / min, vacuum degree -0.09~-0.1MPa, control water temperature 80~90°C to distill the water to dryness, dry at 120°C for 4h to get KOH / A1 2 o 3 42.1g, placed in a desiccator for subsequent use;
[0041] (2) Preparation of imidazole ethanol: Add HFIP 270g, KOH / A1 2 o 3 36g, 19g of imidazole, 44.7g of 1-(2,4-dichlorophenyl)-2-chloro-ethanol, heated to 45°C for 3.5h, followed by TLC, and filtered to obtain filter cake I and filtrate I, filtered Cake I was rinsed with HFIP to obtain lotion I and filter cake II, combined filtrate 1 and lotion 1, and carried out rotary evaporation, controlled rotation speed 70r / min, vacuum degree -0.09~-0.1MPa, water temperature 40~50℃ until no distillation The effluent flows out, add 13g of 10% dilute hydrochloric acid (3.6g of 36% hydrochloric acid mixed with 9.4g of water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com