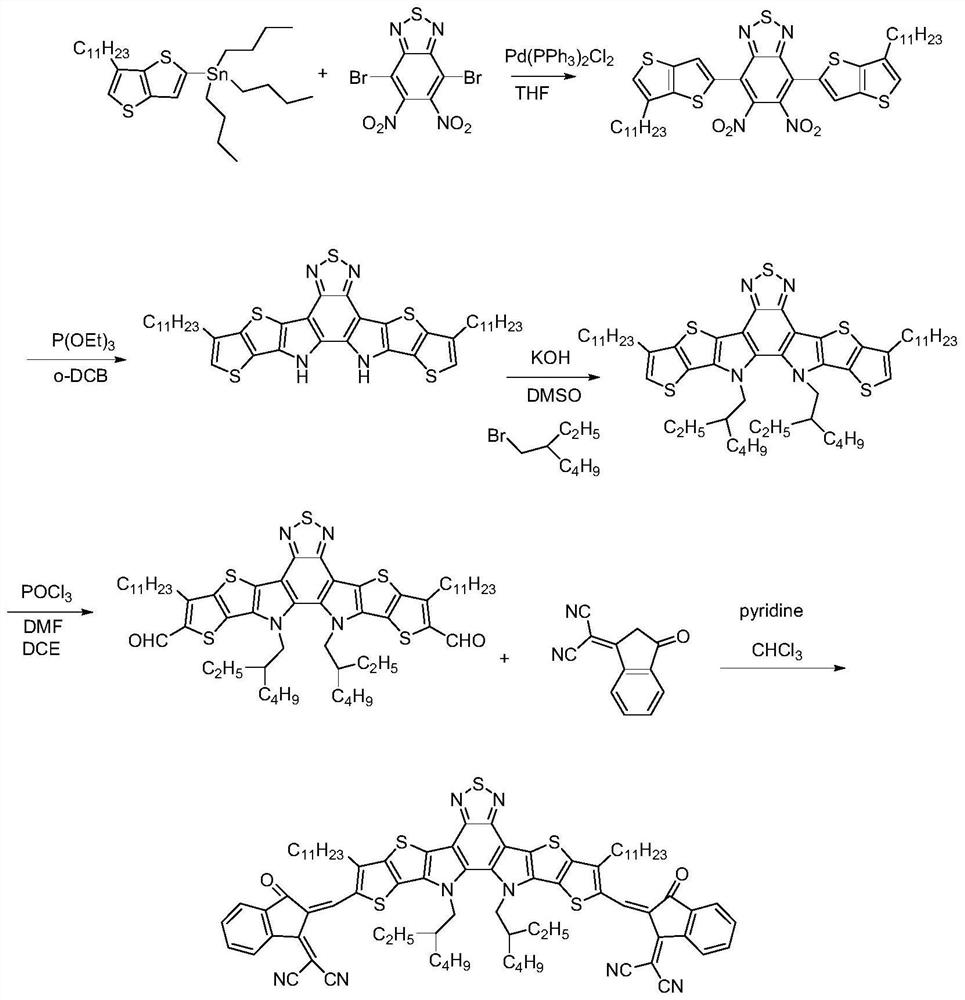
A kind of fused ring benzothiadiazolyl non-fullerene acceptor material and its preparation method and application
A benzothiadiazole non-fullerene and benzothiadiazolyl-based technology, applied in the field of organic solar cell material preparation, can solve the problem of low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] R as mentioned above 1 for for R 3 for EG is When , the preparation of the acceptor material is as follows:
[0079] (1) 4,7-dibromo-5,6-dinitrobenzothiadiazole and compound A obtain compound B through stille coupling reaction:
[0080]
[0081] Synthesis of Compound B: In a 250ml round bottom flask, weigh 4,7-dibromo-5,6-dinitrobenzothiadiazole (7.68g, 20mmol) and tributyl (6-undecylthiophene And[3,2-b]thiophen-2-yl)stannane (25.68g, 44mmol) was dissolved in 100ml of tetrahydrofuran, and bistriphenylphosphinepalladium dichloride (0.62g, 0.88mmol) was added under argon protection in the system. The mixture was refluxed at 80°C for 20 hours. Cooled to room temperature, spin-dried THF, extracted with dichloromethane, spin-dried the solvent to obtain a crude product, separated and purified by silica gel column chromatography to obtain a red solid (10.54 g), which was compound B;
[0082] (2) Compound B, triethyl phosphite and o-dichlorobenzene carry out conde...
Embodiment 2
[0108] R as mentioned above 1 for for R 3 for EG is , the preparation of the acceptor material is as follows: (1) The experimental procedure is basically the same as that of Example 1, and Compound E is prepared according to the experimental procedure of Example 1; (2) Compound E and 5,6-difluoro-3- (Dicyanomethylene) indoketone is obtained ZYQ4 acceptor material by Knoevenagel reaction:
[0109]
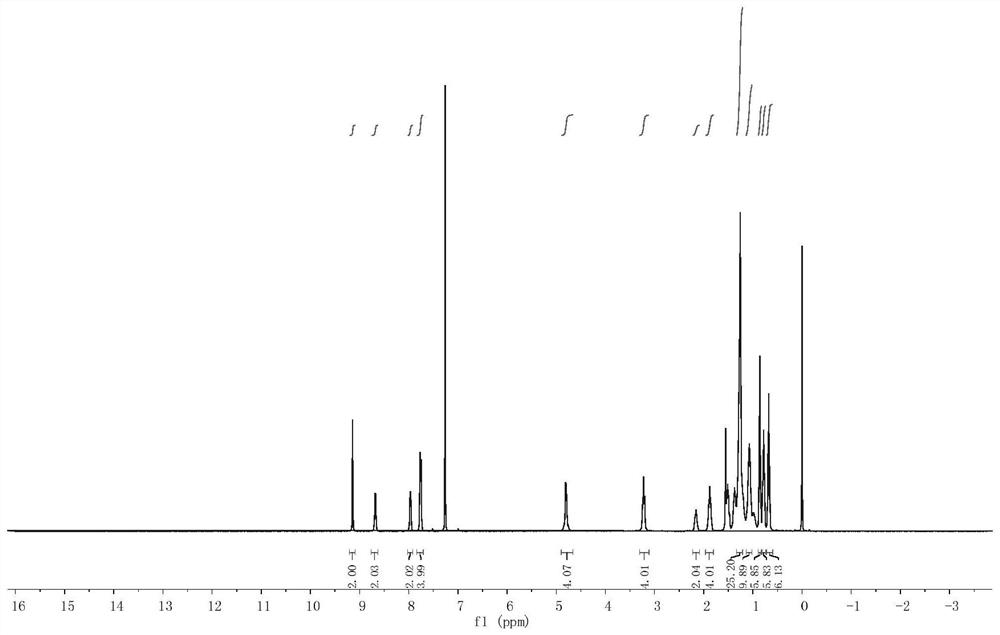
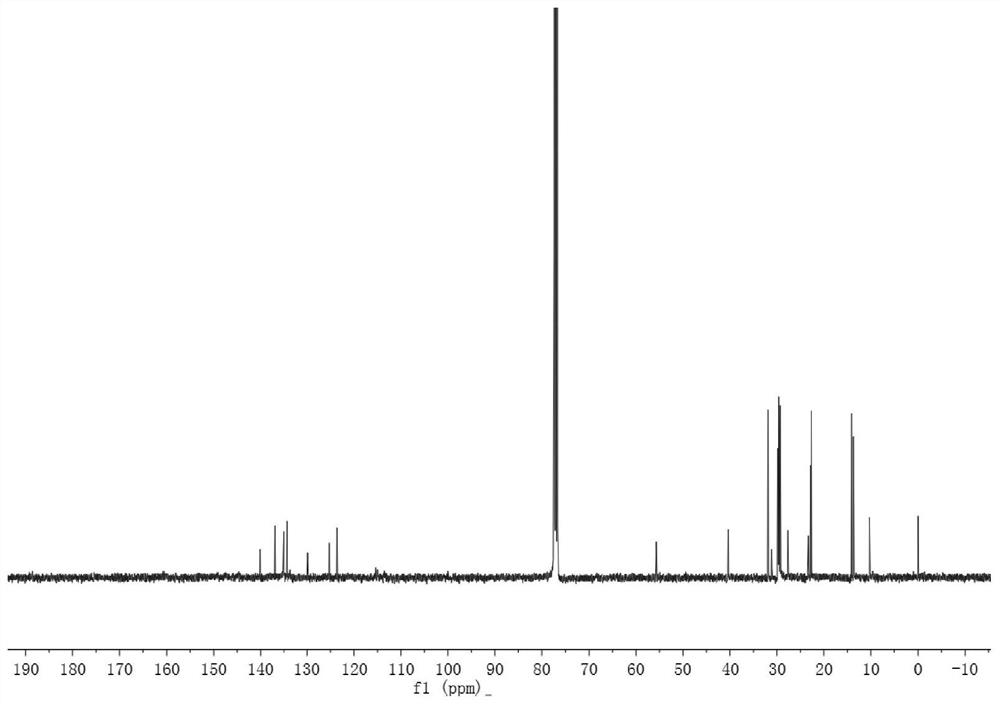
[0110] Synthesis of acceptor material ZYQ4: in a 250ml round bottom flask, compound E (0.154g, 0.15mmol) and 3-(dicyanomethylene) indoketone 0.345g, 1.50mmol) were dissolved in 45ml chloroform, added 1ml of pyridine, the mixed solution was refluxed for 12 hours under the protection of argon, cooled to room temperature, poured into 200ml of anhydrous methanol, and suction filtered to obtain the crude product, which was separated and purified by silica gel column chromatography to obtain a dark blue solid (0.140g). That is, the acceptor material ZYQ4.
[0111] The yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com