Hydrogen peroxide responding type targeted fluorescent medicine-carrying nanomaterial and preparation method
A technology of hydrogen peroxide and drug-loaded nanometers, which is applied in the field of nanomaterials, can solve the problems of lack of application of nanometer drug-loaded systems, and achieve the effects of convenient drug administration monitoring, retaining biocompatibility, and improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
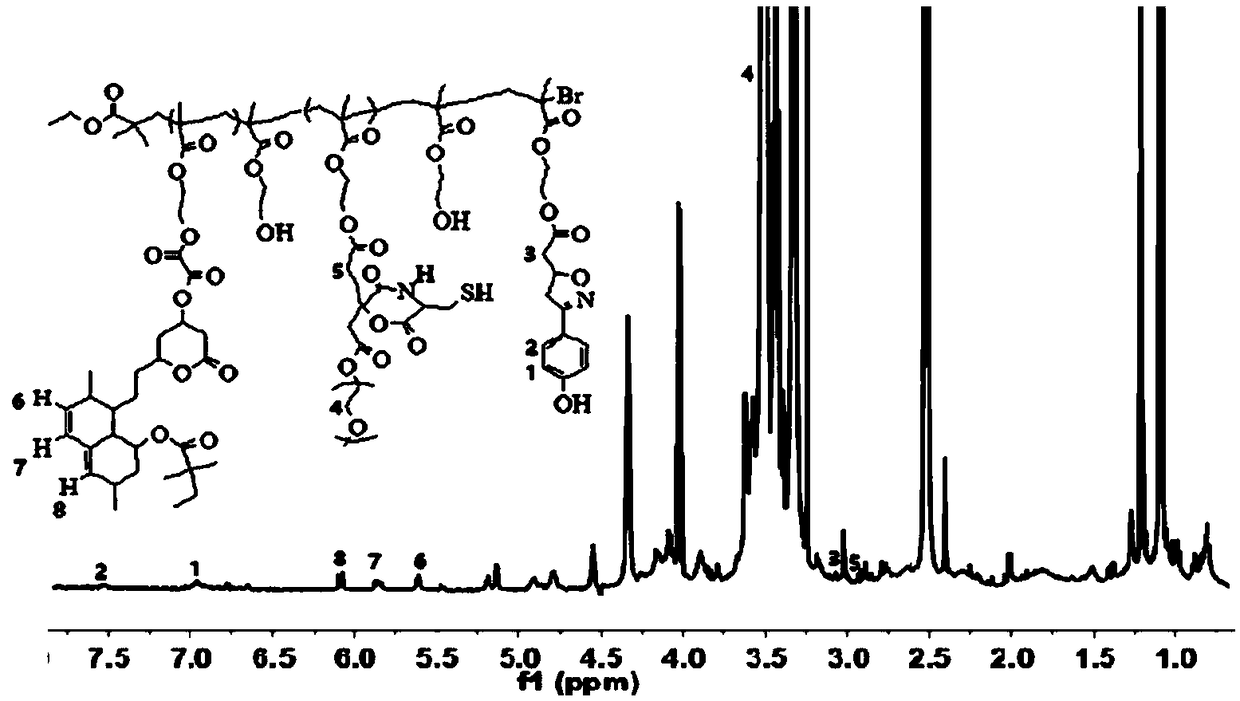
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of polyhydroxyethyl methacrylate (PHEMA)
[0031] Poly(hydroxyethylmethacrylate) (PHEMA) was synthesized by ATRP method. 2-Bipyridine (0.06g), CuCl (0.28g), 4mL N,N'-dimethylformamide, 4mL methyl Hydroxyethyl acrylate and 0.15 mL 2-bromo-2 methacrylate. Carry out vacuum pumping and argon filling operation again to ensure that the reaction system is in an oxygen-free state. Place the check bottle in an oil bath at 70°C for 6 hours. After the reaction is terminated, dissolve the mixture in methanol and use Al 2 o 3 The neutral column removes impurities, and the filtrate is concentrated by rotary evaporation, and precipitated in ether, and then centrifuged, dried and other post-treatments to obtain white powder PHEMA.
Embodiment 2
[0032] Embodiment 2: the synthesis of fluorescent polyethylene glycol (FPEG)
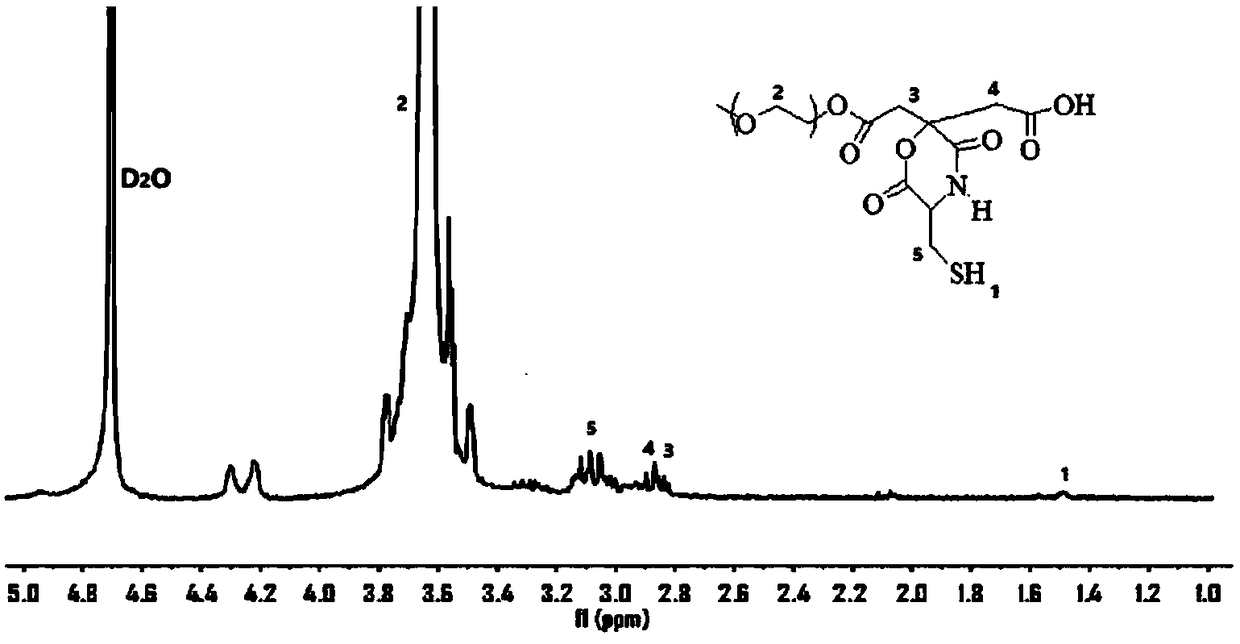
[0033] Take anhydrous citric acid (0.96g), polyethylene glycol monomethyl ether 2000 (2g) and cysteine (0.6g) into a pre-dried 50mL round-bottomed flask, repeatedly pump argon into the bottle for 3 Make sure that the oxygen is completely removed. Afterwards, the mixture was melted at 140° C. and reacted for 2 hours, and the temperature was lowered to 120° C. to continue the reaction for 1 hour. After the reaction, the product was dissolved in deionized water, dialyzed with a dialysis bag with a molecular weight cut off of 2000 for one week, and dried by rotary evaporation to obtain yellow waxy solid FPEG. From figure 1 It can be seen from the NMR images of the target product that each H position and peak area are well assigned. The fluorescent polyethylene glycol prepared in this embodiment presents yellow fluorescence under the irradiation of ultraviolet light, as Figure 4 shown (figure is i...
Embodiment 3
[0034] Embodiment 3: Synthesis of hydrogen peroxide responsive simvastatin polymer (PHEMA-Sim)
[0035] First, simvastatin (1.72g) was dissolved in 20mL of anhydrous dichloromethane, and oxalyl chloride (5.02g) was dissolved in 5mL of anhydrous dichloromethane. Under the condition of ice-salt bath, drop the simvastatin solution into the oxalyl chloride solution at a rate of 1 drop per second, continue to react for 2 hours after the addition, the reaction solution gradually changes from colorless to yellow, and the reaction ends. Subsequently, the solvent and excess oxalyl chloride were removed by rotary evaporation, and 1.84 g of simvastatin acid chloride yellow powder was obtained after vacuum drying, which was re-dissolved in 20 mL of anhydrous dichloromethane. Afterwards, the PHEMA (0.9g) prepared in Example 1 was dissolved in 50mL of anhydrous dichloromethane, and under the protection of argon, the dichloromethane solution containing simvastatin acyl chloride was dropped a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com