A lidocaine gel emplastrum
A technology of lidocaine gel plaster and lidocaine, applied in the direction of medical preparations with non-active ingredients, organic active ingredients, oil/fat/wax non-effective ingredients, etc., can solve the problem of systemic effects and risks Large and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
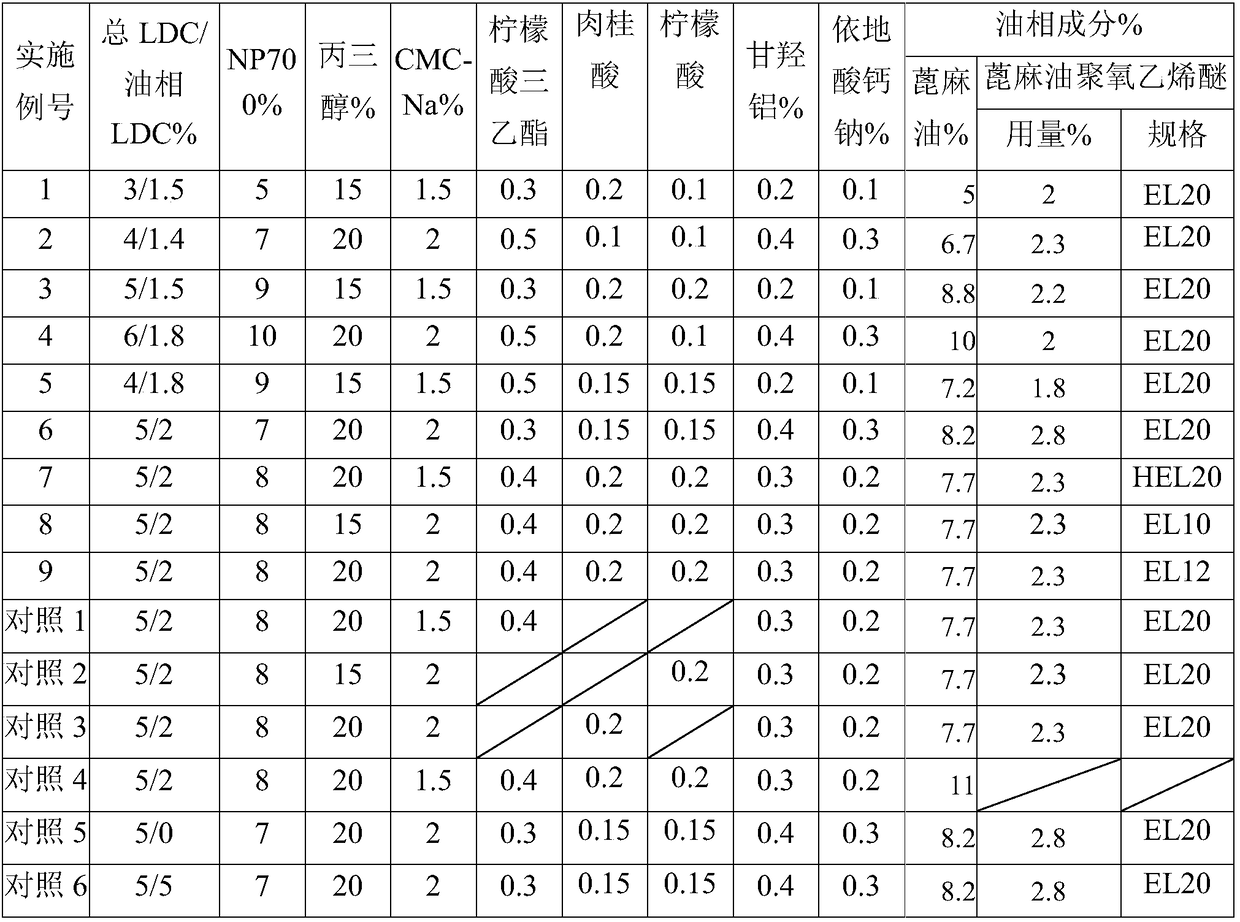
Examples
Embodiment 1
[0019] Pharmacological Example 1, in vitro transdermal experiment
[0020] For details of the method, please refer to the new dosage form of transdermal administration in "New Drug Forms and New Technology" edited by Lu Bin. The modified Franz diffusion cell method was used to use the isolated 3-month-old rat abdomen skin as a barrier, and the bab patches prepared in Examples 1 to 6 and Comparative Examples 1 to 6 were used for in vitro skin penetration tests. The specific experimental methods are:
[0021] Three-month-old healthy rats were put to death by anesthesia, the abdominal hair was removed with scissors, the undamaged skin was removed, and the subcutaneous tissues were removed. After washing, they were fixed to the release ports of the Franz diffusion cell, and pH 7.4 phosphoric acid was added to the receiving chamber. The buffer is used as the release medium to keep the endothelial layer in close contact with the solution. Put the gel patch with the protective layer rem...
Embodiment 2
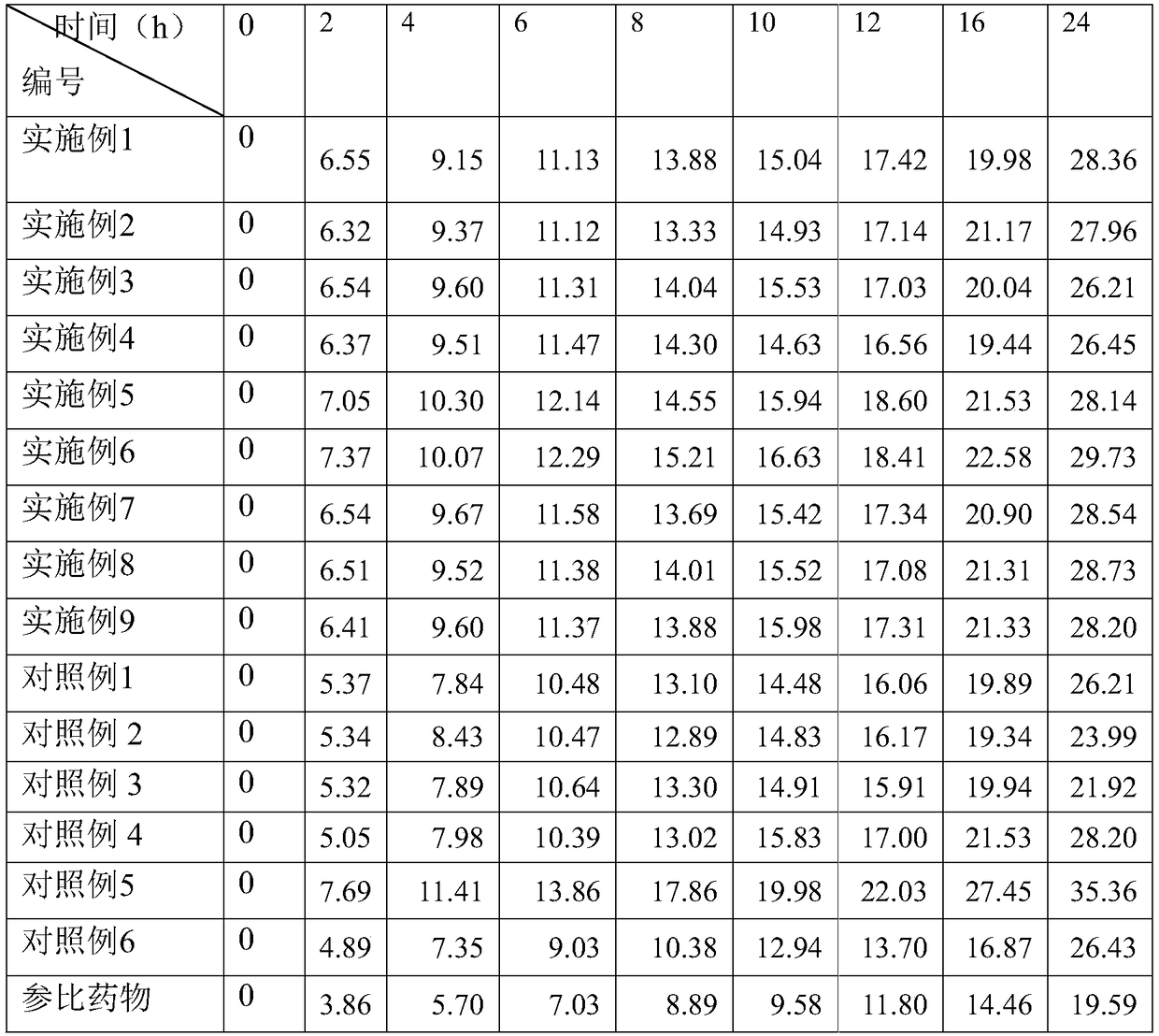
[0024] Pharmacological Example 2, Pharmacokinetic Experiment (Rabbit)
[0025] Take Japanese big-eared white rabbits, weighing 2.0±0.2kg, and randomly divided into groups. Each group uses both male and female, with 8 rabbits in each group. The hair on both sides of the rabbit's back spine was removed with a depilatory one day before the administration. The area of the depilation was about 25cm×15cm. At the same time, it was fasted for 12 hours before administration. The dosage of each group was 50mg / kg, and the patch was applied to both sides of the back spine. Rabbit ears were taken at 15min, 30min, 1, 2, 3, 4, 6, 8, 12, 24h after administration. The marginal venous blood was 2mL, and the blood drug concentration (ng / mL) was detected. The data is expressed in terms of mean±standard deviation, and the data is processed by Excel.
[0026] The grouping and test results are as follows (ng / mL) (n=8, means±s)
[0027]
[0028]
[0029] The experimental results show that, compared wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com