Use of rimantadine Schiff base
A technology of rimantadine Schiff base and rimantadine is applied in the directions of antiviral agents, antibacterial drugs, drug combinations, etc., to achieve the effects of less by-products, mild reaction conditions, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
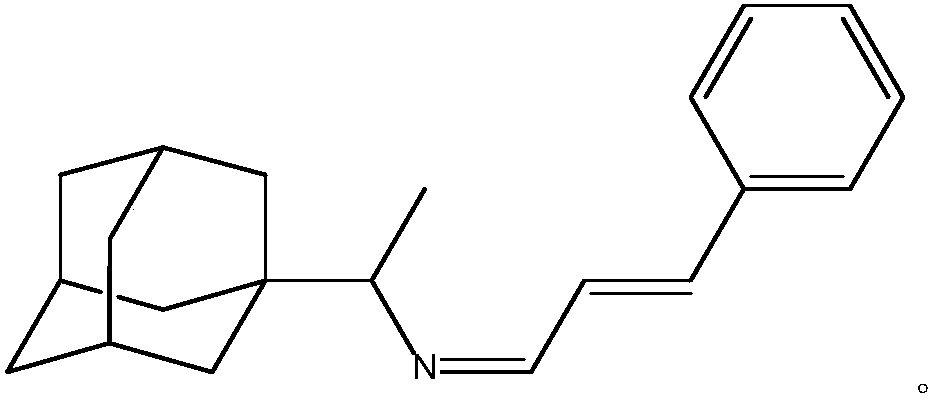
[0025] A kind of purposes of rimantadine Schiff base, the prepared rimantadine Schiff base is rimantadine cinnamaldehyde Schiff base, its stereoisomer or pharmaceutically accessible salt, the chemical structural formula is:
[0026]
[0027] A kind of purposes of rimantadine Schiff base, the synthetic method of prepared rimantadine Schiff base comprises the steps:
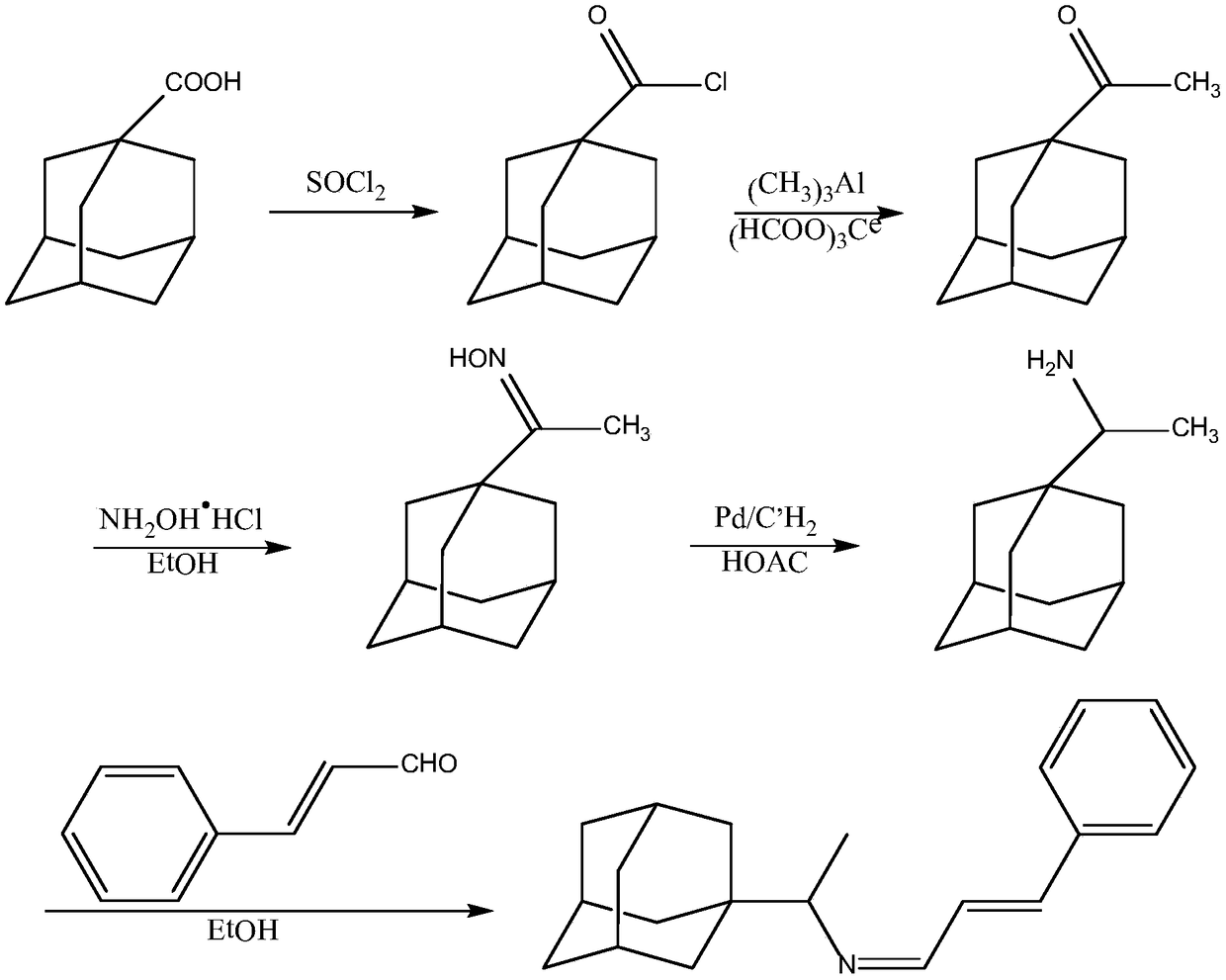
[0028] Preparation of adamantanecarboxylic acid chloride: Add 1.8g of adamantanecarboxylic acid and 3.6mL of thionyl chloride into a three-necked flask, heat to 80°C, reflux for 2 hours, recover thionyl chloride by vacuum distillation, and then add 5mL×2 of benzene for extraction. Gained extract is directly used for next step reaction;
[0029] Preparation of adamantanemethyl ketone: Add 0.10g trimethylaluminum and 0.01g cerium formate to a three-necked flask, then add a benzene solution of adamantanecarbonyl chloride dropwise at 0.25mL / min to make adamantanecarbonyl chloride and trimethylaluminum The reaction ...
Embodiment 2
[0034] A kind of purposes of rimantadine Schiff base, the prepared rimantadine Schiff base is rimantadine cinnamaldehyde Schiff base, its stereoisomer or pharmaceutically accessible salt, the chemical structural formula is:
[0035]
[0036] A kind of purposes of rimantadine Schiff base, the synthetic method of prepared rimantadine Schiff base comprises the steps:
[0037] Preparation of adamantanecarboxylic acid chloride: Add adamantanecarboxylic acid and thionyl chloride into a three-necked flask, heat to 80°C, reflux for 2 hours, recover thionyl chloride by vacuum distillation, then add benzene for extraction, and the obtained extract is directly used in the following one step reaction;
[0038]Preparation of adamantane methyl ketone: Add trimethylaluminum and cerium formate to a three-necked flask, and then drop a benzene solution of adamantanecarbonyl chloride at 0.1mL / min to react adamantanecarbonyl chloride and trimethylaluminum to form adamantane Methyl ketone, aft...
Embodiment 3
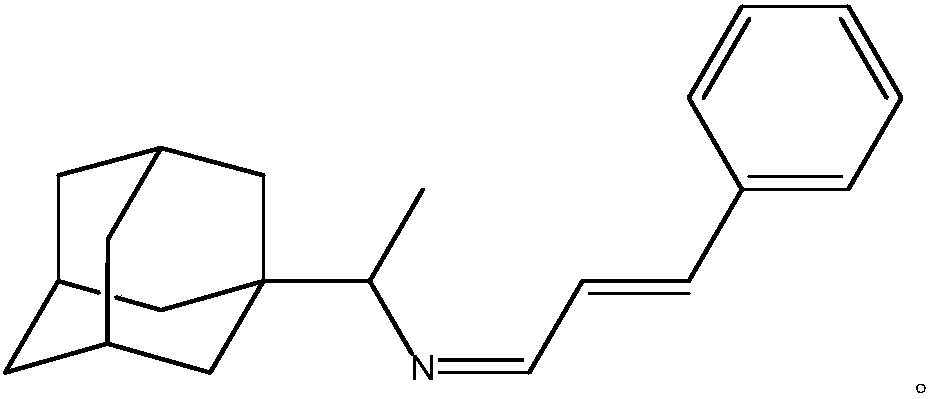
[0043] A kind of purposes of rimantadine Schiff base, the prepared rimantadine Schiff base is rimantadine cinnamaldehyde Schiff base, its stereoisomer or pharmaceutically accessible salt, the chemical structural formula is:
[0044]
[0045] A kind of purposes of rimantadine Schiff base, the synthetic method of prepared rimantadine Schiff base comprises the steps:
[0046] Preparation of adamantanecarboxylic acid chloride: Add adamantanecarboxylic acid and thionyl chloride into a three-necked flask, heat to 80°C, reflux for 2 hours, recover thionyl chloride by vacuum distillation, then add benzene for extraction, and the obtained extract is directly used in the following one step reaction;
[0047] Preparation of adamantane methyl ketone: Add trimethylaluminum and cerium formate to a three-necked flask, and then drop a benzene solution of adamantanecarbonyl chloride at 0.5mL / min to react adamantanecarbonyl chloride and trimethylaluminum to form adamantane Methyl ketone, af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com