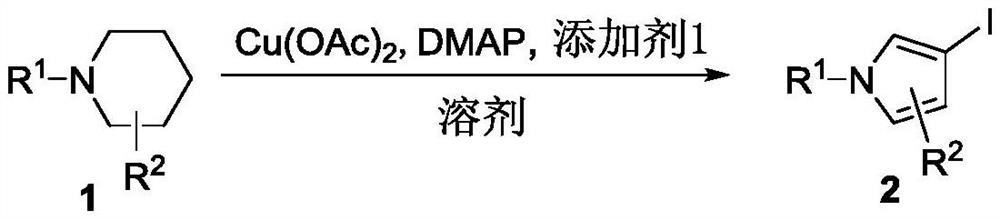
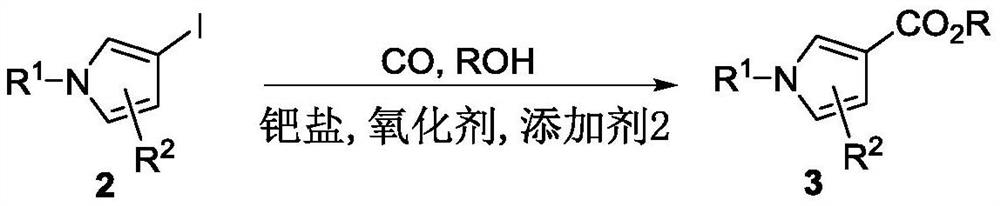
A kind of synthetic method of pyrrole carboxylate compound
A technology of ester compound and pyrrole carboxylic acid, which is applied in the direction of organic chemistry, can solve the problems of poor reaction regioselectivity, difficult to obtain substrate, long synthetic route, etc., and achieve the effect of simple operation, wide application range and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
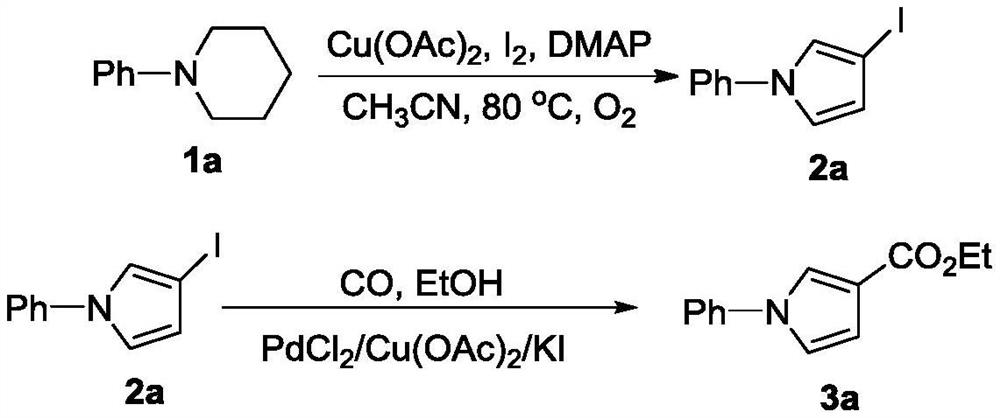
[0023] Add 1a (0.5mmol, 81mg), acetonitrile (5mL), anhydrous copper acetate (1mmol, 181mg), iodine (0.5mmol, 127mg) and 4-dimethylaminopyridine (DMAP, 0.5 mmol, 61 mg), vacuumized and filled with oxygen (1 atm), placed in an oil bath at 80°C and stirred for 10 h. Then, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate. Filter, spin dry, and separate through silica gel column (petroleum ether / ethyl acetate=100 / 1) to obtain compound 2a (87mg, 65%). 2a (0.3mmol, 81mg), PdCl 2 (0.03mmol, 5.3mg), copper acetate (0.3mmol, 54mg) and potassium iodide (0.3mmol, 50mg) were placed in a 10mL Shrek tube, evacuated and filled with CO, and then ethanol (3mmol, 175μL) and acetonitrile (3mL) Add it to the system, raise the temperature to 80°C, and react for 12h. The reaction system was post-treated to obtain the target product 3a (45 mg, 70%). The charac...
Embodiment 2
[0025] Add 1a (0.5mmol, 81mg), acetonitrile (5mL), anhydrous copper acetate (0.5mmol, 91mg), iodine element (0.125mmol, 32mg) and DMAP (0.5mmol, 61mg) to a 10mL Shrek tube successively, and draw After inflating oxygen (1 atm) under vacuum, it was placed in an oil bath at 80° C. and stirred for 10 h. Then, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate. Filter, spin dry, and separate through silica gel column (petroleum ether / ethyl acetate=100 / 1) to obtain compound 2a (34 mg, 25%). According to the method of Example 1, 2a can be transformed into 3a.
Embodiment 3
[0027] Add 1a (0.5mmol, 81mg), acetonitrile (5mL), anhydrous copper acetate (0.5mmol, 91mg), iodine element (0.25mmol, 64mg) and DMAP (0.5mmol, 61mg) to a 10mL Shrek tube successively, and draw After inflating oxygen (1 atm) under vacuum, it was placed in an oil bath at 80° C. and stirred for 10 h. Then, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), and the organic phases were combined and dried over anhydrous sodium sulfate. Filter, spin dry, and separate through silica gel column (petroleum ether / ethyl acetate=100 / 1) to obtain compound 2a (69 mg, 51%). According to the method of Example 1, 2a can be transformed into 3a.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com