Mass control method of Jichuan decoction preparation
A quality control method and preparation technology, applied in the field of medicine, can solve the problems of long analysis time, insufficient comprehensiveness, and few common peaks, etc., and achieve the effects of shortening analysis time, good quality of medicinal materials, and cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
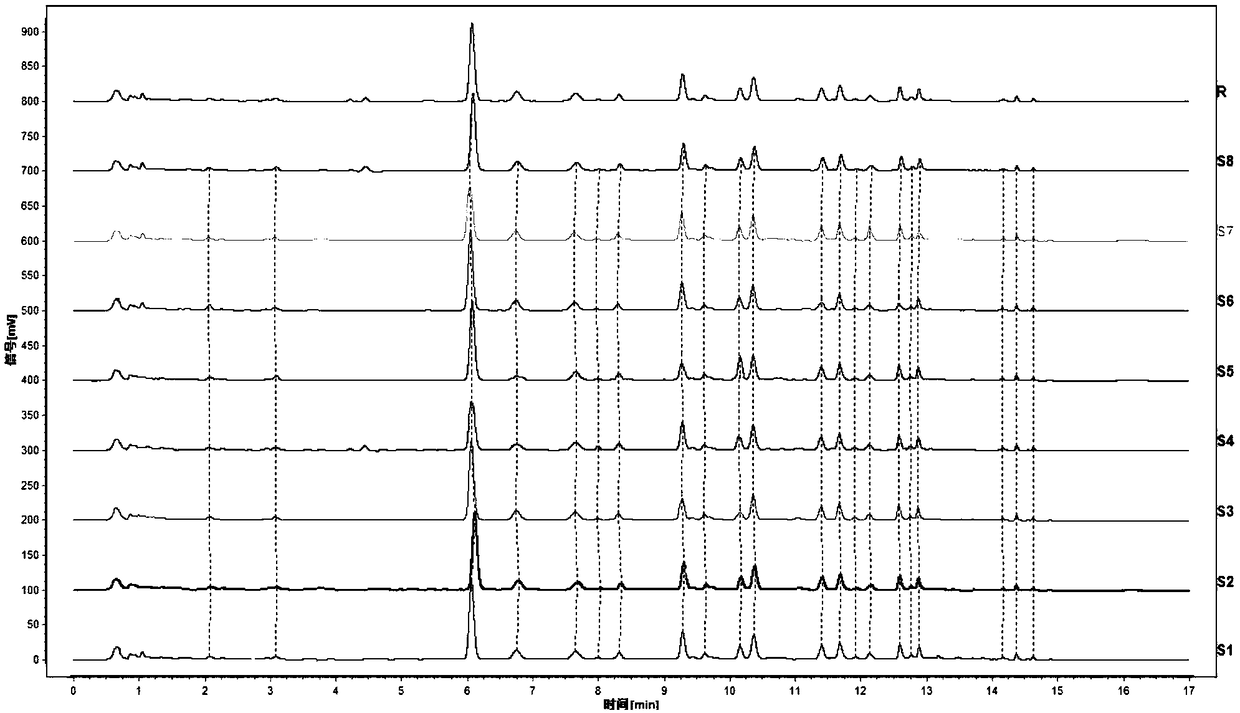
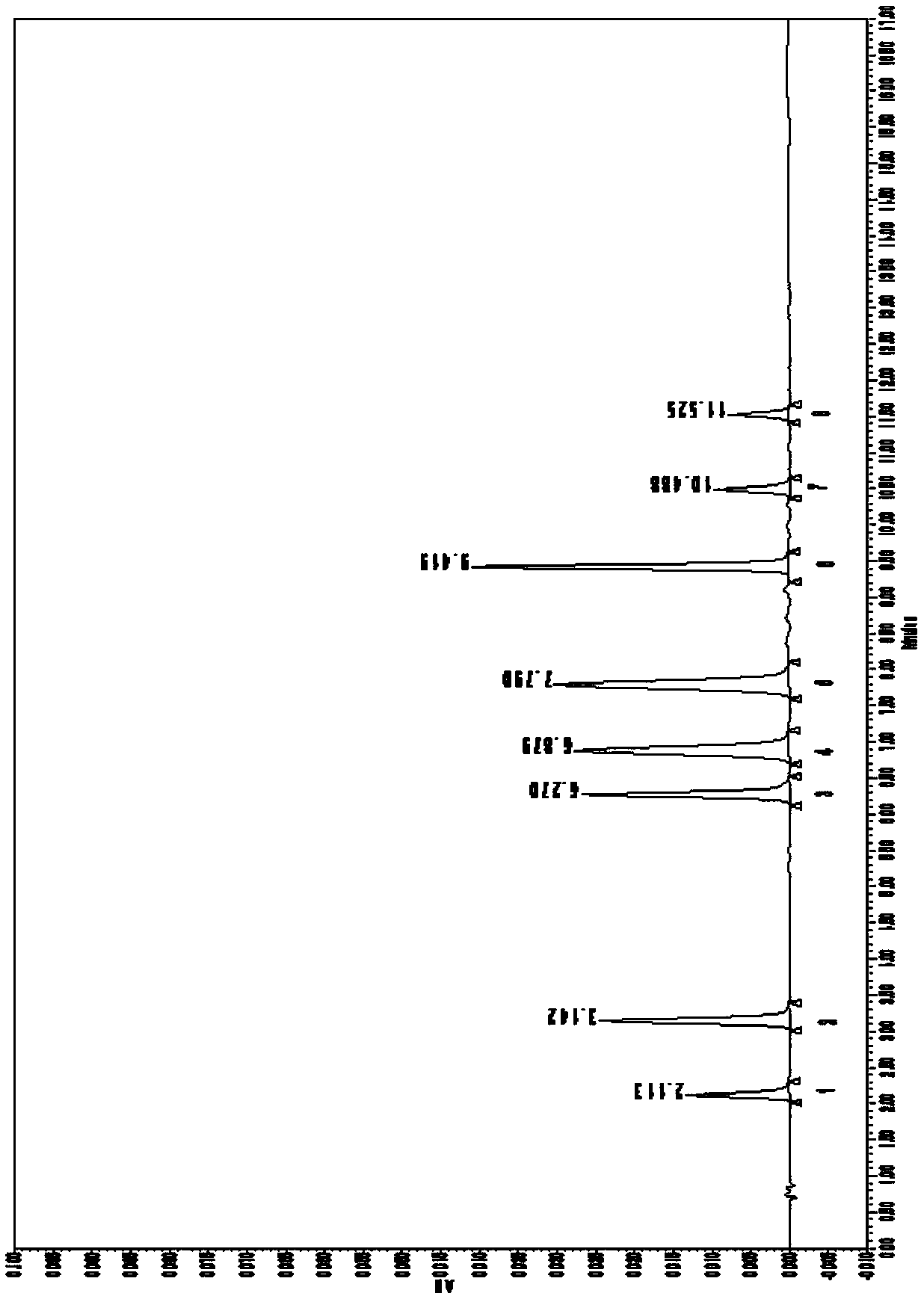
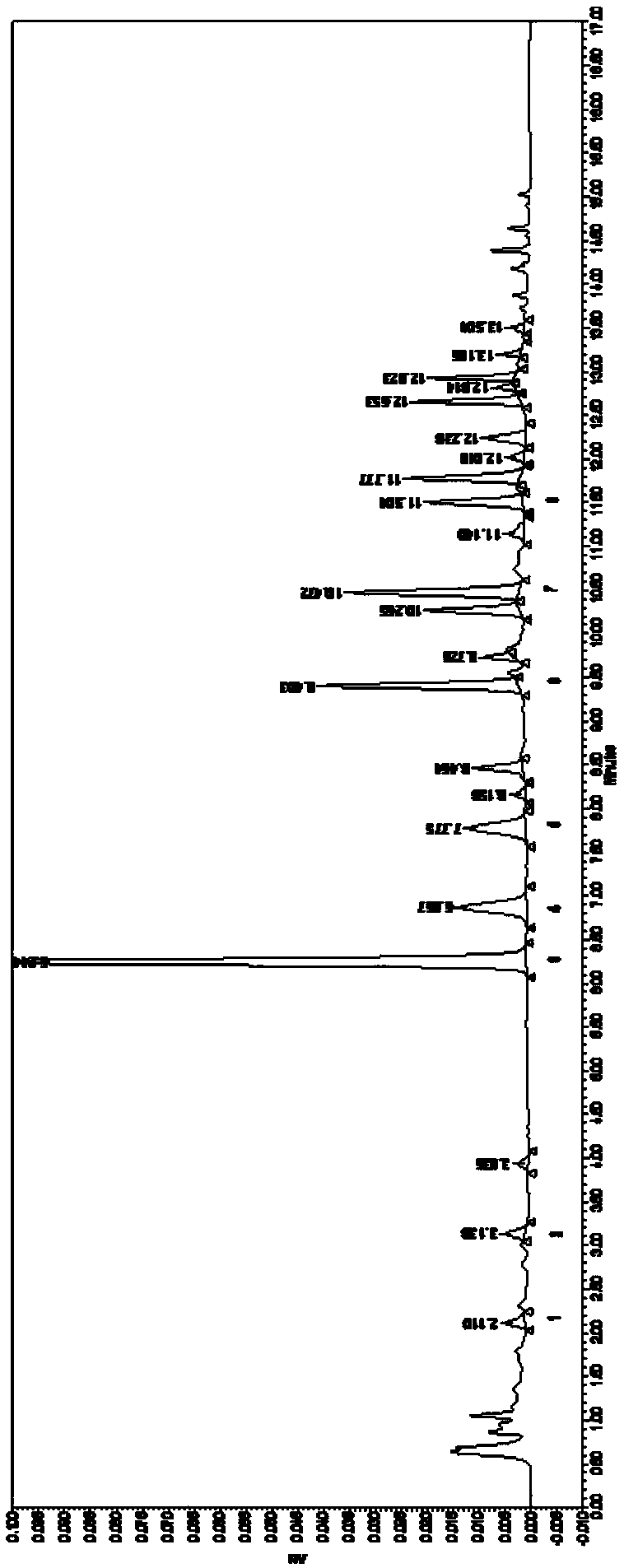
[0064] Fingerprint of Jichuan Decoction
[0065] 1. Instruments and reagents
[0066] Ultra-high performance liquid chromatography (Waters Acquity H-Class), analytical balance (METTLER TOLEDO XS / 205DU), ultrasonic cleaning machine (KQ-5200E);
[0067] Chromatographic column: C18 column (Waters ACQUITY UPLC HSS T3 (2.1*50mm, 1.8μm);
[0068] Reagents: Acetonitrile is chromatographically pure, water is ultrapure water; other reagents are analytically pure, and reference materials are purchased from China National Institute for Food and Drug Control;
[0069] Reagent: Jichuanjian Granules (batch number: 17090401-17090408);
[0070] 2. Chromatographic conditions: use 0.1% formic acid aqueous solution as mobile phase A, acetonitrile as mobile phase B, gradient elution conditions are 0~1.04min, 15%B, 1.04~5.60min, 15%–16%B, 5.60~ 10.42min, 16%–25%B, 10.42~13.55min, 25%–95%B, 13.55~13.76min, 95-15%B, 13.76~20min, 15%B. The flow rate is 0.2ml / min; the column temperature is 25°C; t...
Embodiment 2
[0084] Fingerprint of Jichuan Decoction
[0085] 1. Instruments and reagents
[0086] Ultra-high performance liquid chromatography (Waters Acquity H-Class), analytical balance (METTLER TOLEDO XS / 205DU), ultrasonic cleaning machine (KQ-5200E);
[0087] Chromatographic column: ACQUITY UPLC BEH C18 (2.1*50mm, 1.7μm);
[0088] Reagents: Acetonitrile is chromatographically pure, water is ultrapure water; other reagents are analytically pure, and reference materials are purchased from China National Institute for Food and Drug Control;
[0089] Reagent: Jichuanjian Granules (batch number: 17090401-17090408);
[0090] 2. Chromatographic conditions: use 0.1% phosphoric acid aqueous solution as mobile phase A, acetonitrile as mobile phase B, gradient elution conditions are 0~1.04min, 12%B, 1.04~5.60min, 12%–16%B, 5.60~ 10.42min, 16%–24%B, 10.42~13.55min, 24%–95%B, 13.55~13.76min, 95-12%B, 13.76~17min, 12%B. The flow rate is 0.5ml per minute; the column temperature is 35°C; the dete...
Embodiment 3
[0098] Fingerprint of Jichuan Decoction
[0099] 1. Instruments and reagents
[0100] Ultra-high performance liquid chromatography (Waters Acquity H-Class), analytical balance (METTLER TOLEDO XS / 205DU), ultrasonic cleaning machine (KQ-5200E);
[0101] Chromatographic column: Aglient Poroshell 120EC-C18 (3.0*50mm, 2.7μm);
[0102] Reagents: Acetonitrile is chromatographically pure, water is ultrapure water; other reagents are analytically pure, and reference materials are purchased from China National Institute for Food and Drug Control;
[0103] Reagent: Jichuanjian Granules (batch number: 17090401-17090408)
[0104] 2. Chromatographic conditions: use 0.1% acetic acid aqueous solution as mobile phase A, acetonitrile as mobile phase B, gradient elution conditions are 0-2min, 10-13% B, 2-6.25min, 13%-18% B, 6.25 ~10.42min, 18%–24%B, 10.42~13.55min, 24%–95%B, 13.55~13.76min, 95%–10%B, 13.76~17min, 10%B. The flow rate is 0.3ml per minute; the column temperature is 30°C; the de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com