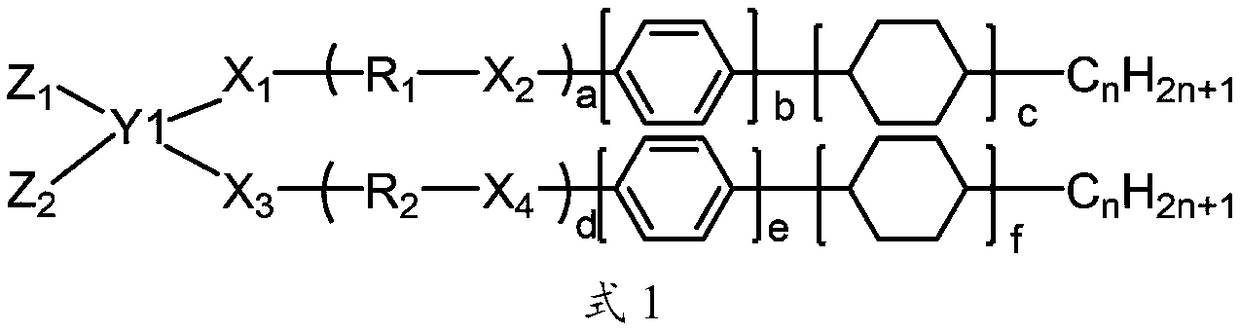
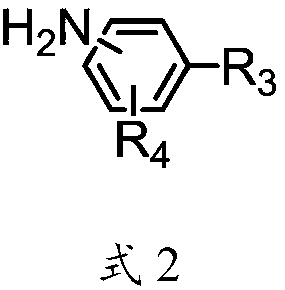
Liquid crystal aligning agent, liquid crystal aligning membrane and liquid crystal display element
A technology of liquid crystal alignment agent and solvent, which is applied in the direction of liquid crystal materials, coatings, instruments, etc., to achieve the effects of increasing anchoring ability, reducing electrode stripes, and reducing electrostatic breakdown
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
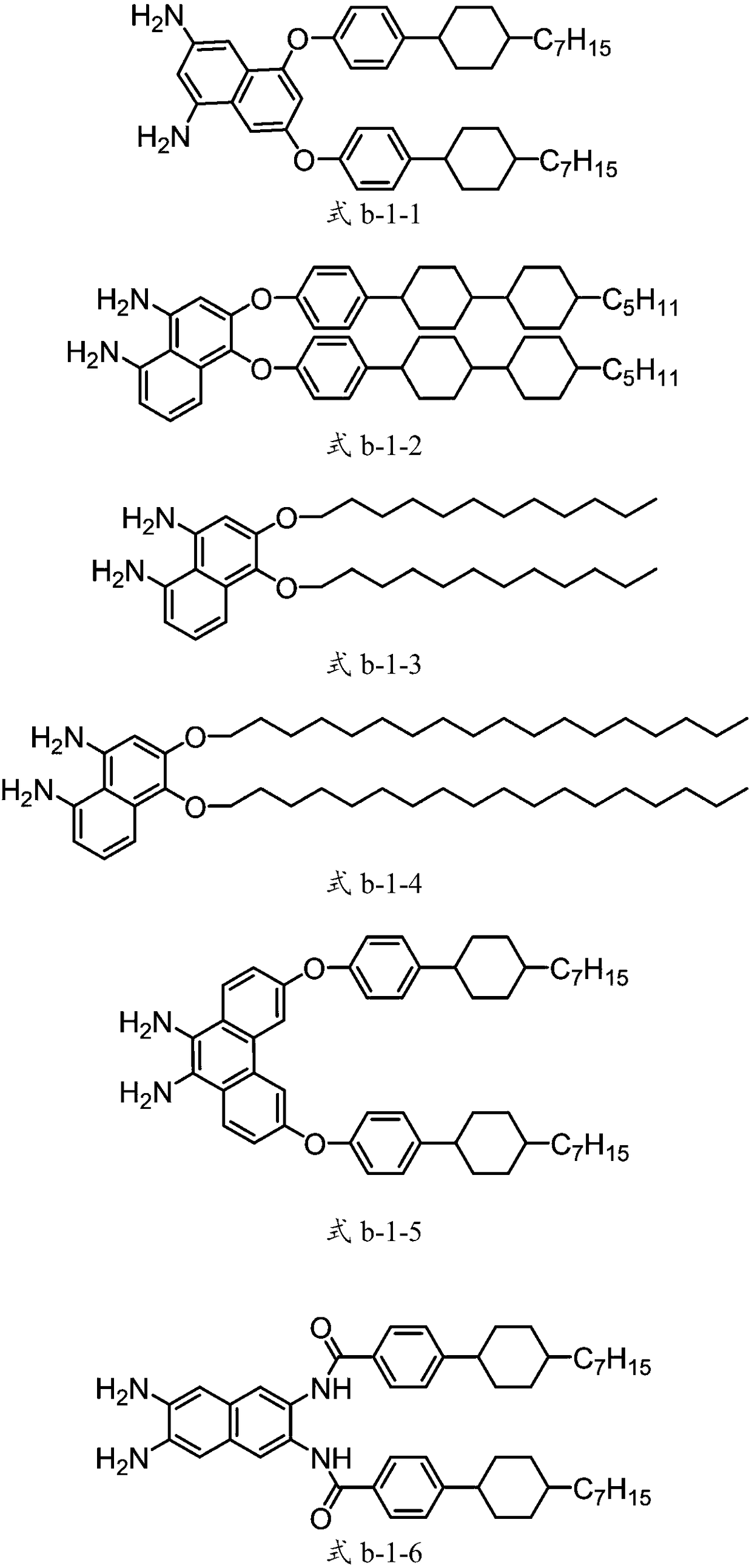
[0047] The synthesis of the diamine compound represented by structural formula b-1-1 is shown in Synthetic Route 1:
[0048]
[0049] The specific operation process is:
[0050] (1) Synthesis of compound b-1-1a
[0051] Put 1,3-dibromo-5,7-dinitronaphthalene (37.6g, 0.1mol) and 4-(4-heptylcyclohexyl)phenol (57.63g, 0.21mol) into a 1000mL three-neck round bottom flask , anhydrous potassium carbonate powder (41.46g, 0.3mol) and 300g toluene, the temperature of the oil bath was raised to 105°C and stirred for 3 hours, followed by TLC until there was no remaining raw material 1,3-dibromo-5,7-dinitronaphthalene , lower the system to room temperature, stop stirring, transfer the reaction solution to a separatory funnel and wash it with water until neutral, remove the solvent toluene to obtain a yellow solid, add 228g of absolute ethanol and 114gTHF to it and stir for 30min, then suction filter, dry and filter 65.62g of yellow crystals were obtained from the cake, and the produc...
Embodiment 2~5
[0056] The compounds represented by the structural formulas (b-1-2) to (b-1-5) can be etherified and nitro-dihalogenated arene compounds and their respective parent phenolic compounds according to "synthetic route 1" Catalytic hydrogenation reaction. The specific yield, high-resolution mass spectrometry results and elemental analysis results of each product are shown in Table 1 below.
[0057] Yield, mass spectrum, elemental analysis data of each compound in Table 1 embodiment 2~5
[0058]
Embodiment 6
[0060] The synthesis of the compound represented by structural formula b-1-6 is shown in Synthetic Route 2:
[0061]
[0062] The specific operation process is as follows:
[0063] (1) Synthesis of compound b-1-6a
[0064] Add 4-(4-heptylcyclohexyl)benzoic acid (30.25g, 0.10mol), thionyl chloride (35.69g, 0.3mol) and 250g toluene and 3 drops of DMF into a 500mL three-neck round bottom flask, and heat up to reflux React for 5 hours, then distill out the residual thionyl chloride and toluene in the system. After the system is evaporated to dryness, it is cooled to room temperature to obtain a brownish-yellow solid sticky substance. Add 200g of anhydrous toluene to the system and stir to completely dissolve the solid to obtain b - 1-6a in toluene.
[0065] (2) Synthesis of compound b-1-6b
[0066] The compound 2,3-diamino-6,7-dinitronaphthalene (11.17g, 45mmol), triethylamine (12.14g, 120mmol) and 300g of toluene were put into a 1L three-necked flask, and the temperature of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com