Method for establishing disease model of 'human-derived' short QT syndrome
A technology for QT syndrome and disease model, which is applied in the establishment of "human-derived" short QT syndrome disease models, can solve problems such as endogenous gene expression changes and mutations, and achieve accurate research results and increased current density. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
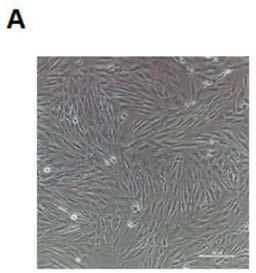
[0043] Example 1: Acquisition and cultivation of SQTs patient fibroblasts
[0044] Disinfect the skin surface of the patient with povidone iodine, put the skin piece into the prepared DMEM culture solution under aseptic conditions immediately after removing the skin piece, place the centrifuge tube containing the skin piece on the test tube rack of the ultra-clean bench, and prepare a large 60mm cell culture dish, add 5ml DPBS, transfer the skin piece to the Petri dish for rinsing. Cut the skin block into small pieces of about 1 mm to facilitate the digestion of collagenase. Equipped with 1mg / ml type Ⅰ collagenase solution. Prepare two 1.5ml centrifuge tubes, add about 1.0ml of collagenase solution to each tube, and evenly transfer the cut skins into the centrifuge tubes. Cover the centrifuge tube tightly and place it obliquely in a 37°C incubator, start timing, and place the culture solution at room temperature for later use. After 6 hours of enzyme digestion, take out the...
Embodiment 2
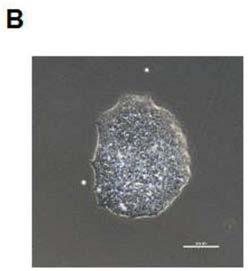
[0045] Example 2: Establishment of SQTs patient-specific iPSC strains
[0046] 2 days before transfection, prepare 1 × 10 6 Skin fibroblasts from patients with SQTs are guaranteed to reach a density of 50-80% on the day of transfection. The cells were replaced with culture medium 1 day before transfection. On the day of transfection, the cells were replaced with 1ml culture medium. Take out an aliquot of Sendai virus from the -80 degree refrigerator, melt it on ice, add it to the cells after it is completely dissolved, and mix it repeatedly. After 24 hours of transfection, the virus was removed and the medium was replaced with fresh culture medium. On day 2 of transfection, no treatment was performed. The culture medium was changed every other day until the 6th day of transfection. On the 7th day of transfection, aspirate the culture medium, rinse the cells with DPBS, digest the cells with 0.25ml TrypLE, centrifuge, and then resuspend the cells in the culture medium at 1x...
Embodiment 3
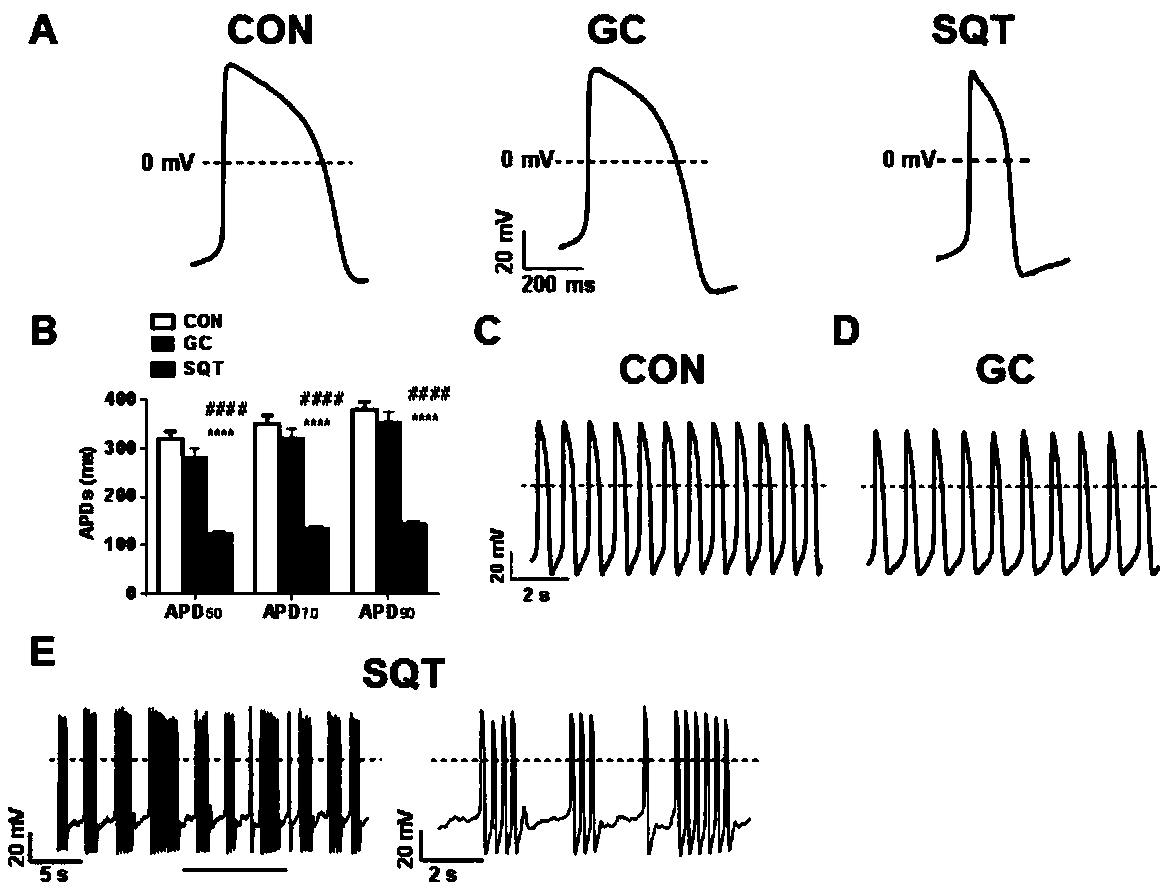
[0047] Example 3: Generation of SQTs patient-specific cardiomyocytes
[0048]The successfully established iPS cell line begins to differentiate into cardiac muscle at about the 20th passage. First, SQTs patient-specific iPSCs were inoculated into 6-well plates at a ratio of 1:8, and the mTeSR medium was replaced every day. When the cell density reached about 80%, the differentiation operation could be performed. On the first day of differentiation, suck up the old medium, wash it once with myocardial differentiation medium (RPMI+B27-Insulin), add 2ml of differentiation medium containing 8μM CHIR to each well, and replace it with myocardial differentiation medium 2 days later, each well On the third day, 2 ml of myocardial differentiation medium containing 5 μM IWR was added to each well, and the culture was continued for 2 days. On day 5, replace the cardiomyocyte differentiation medium. After the 7th day, the culture medium for cardiomyocytes (RPMI+B27+Insulin) was replaced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com