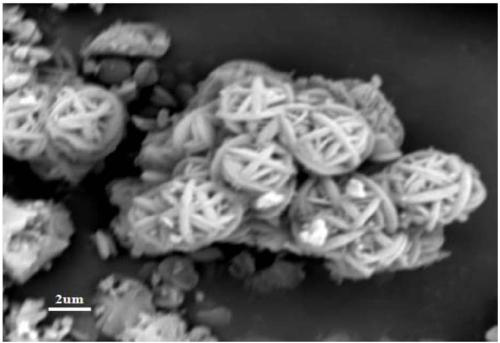
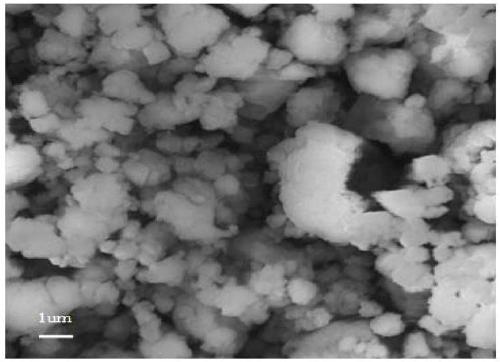
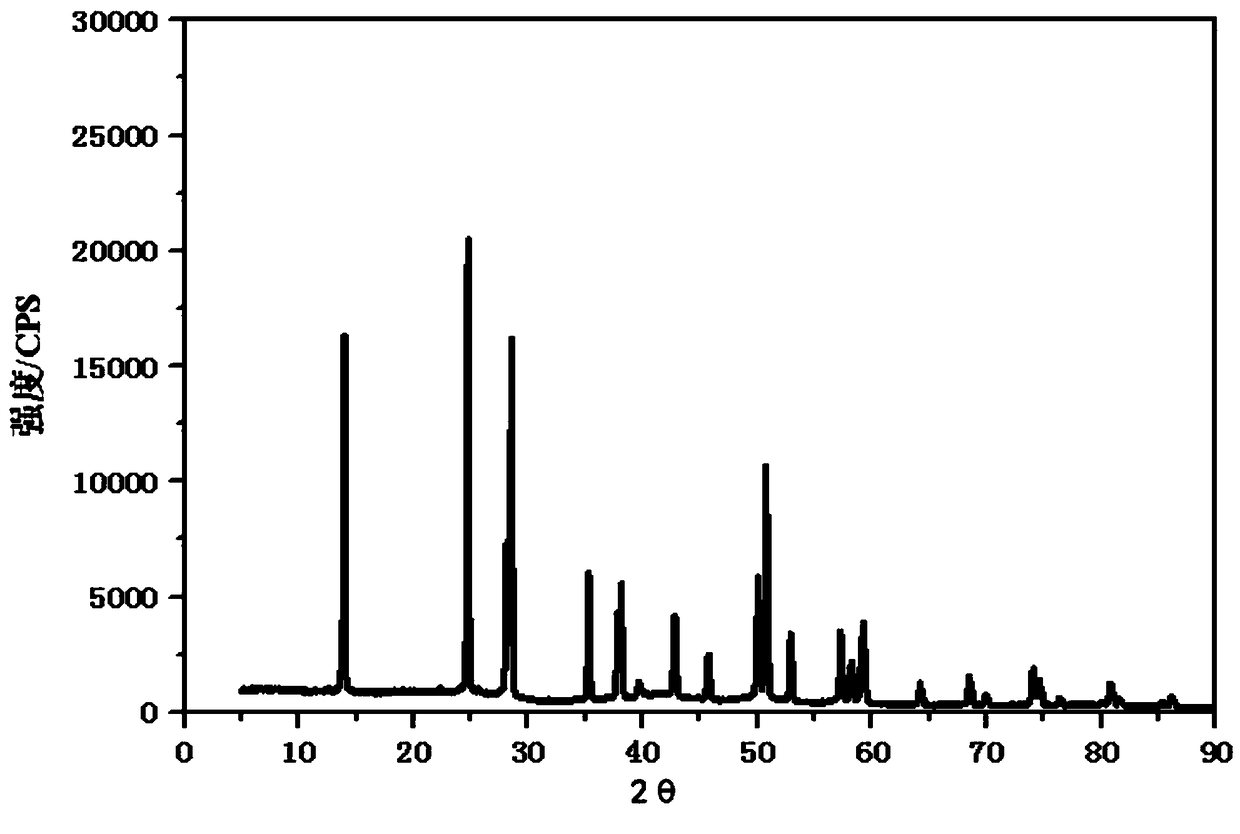
AlF3, and preparation method and application thereof
A technology of aluminum fluoride and aluminum salt, which is applied in the direction of aluminum fluoride, aluminum halide, electrical components, etc., can solve the problems of unfavorable human health, product impurities, toxicity, etc., achieve excellent electrochemical performance, simple preparation process, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A preparation method of aluminum fluoride, comprising the steps:
[0052] 1) Weigh 10g of aluminum nitrate nonahydrate into 30mL of deionized water, stir evenly at 100°C, slowly add 2.96g of ammonium fluoride, and stir to obtain a mixed solution;
[0053] 2) The mixed solution was heated at 80°C for 2h, then transferred to room temperature to stand for crystallization for 10h, suction filtered and washed with ethanol for 3 times to obtain white crystals, and the white crystals were dried in a blast oven at 100°C for 10h to obtain the precursor;
[0054] 3) Place the precursor in a tube furnace, program the temperature to 400°C at 5°C / min, keep the temperature for 3 hours, and naturally cool to room temperature to obtain aluminum fluoride.
Embodiment 2
[0056] A preparation method of aluminum fluoride, comprising the steps:
[0057] 1) Weigh 10g of aluminum nitrate nonahydrate in 30mL of deionized water, stir evenly at 100°C, slowly add 3.94g of ammonium fluoride, and stir to obtain a mixed solution;
[0058] 2) The mixed solution was heated at 80°C for 2h, then transferred to room temperature to stand for crystallization for 10h, suction filtered and washed with ethanol for 3 times to obtain white crystals, and the white crystals were dried in a blast oven at 100°C for 10h to obtain the precursor;
[0059]3) Place the precursor in a tube furnace, program the temperature to 400°C at 5°C / min, keep the temperature for 3 hours, and naturally cool to room temperature to obtain aluminum fluoride.
Embodiment 3
[0061] A preparation method of aluminum fluoride, comprising the steps:
[0062] 1) Weigh 13g of aluminum nitrate nonahydrate into 30mL of deionized water, stir evenly at 100°C, slowly add 3.85g of ammonium fluoride, and stir to obtain a mixed solution;
[0063] 2) The mixed solution was heated at 80°C for 2h, then transferred to room temperature to stand for crystallization for 10h, suction filtered and washed with ethanol for 3 times to obtain white crystals, and the white crystals were dried in a blast oven at 100°C for 10h to obtain the precursor;
[0064] 3) Place the precursor in a tube furnace, program the temperature to 450°C at 5°C / min, keep the temperature for 3 hours, and naturally cool to room temperature to obtain aluminum fluoride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com