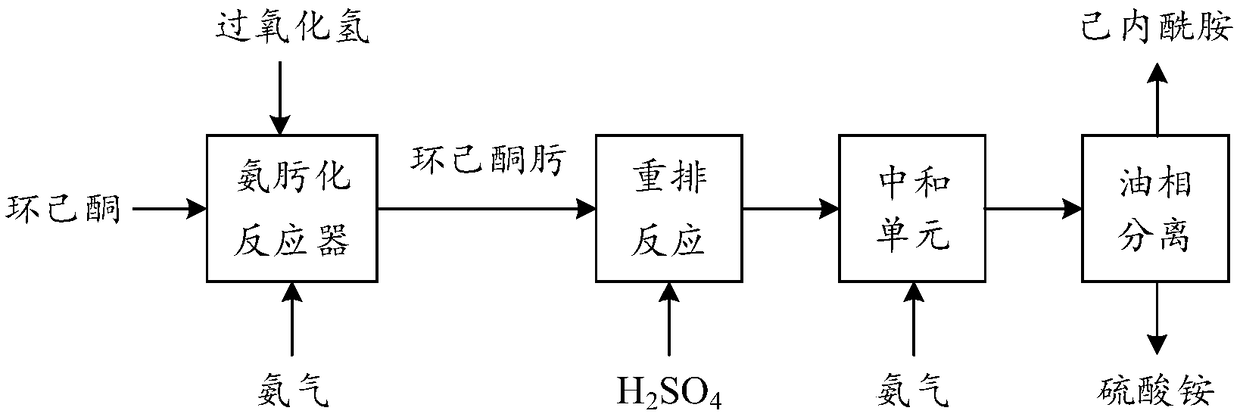
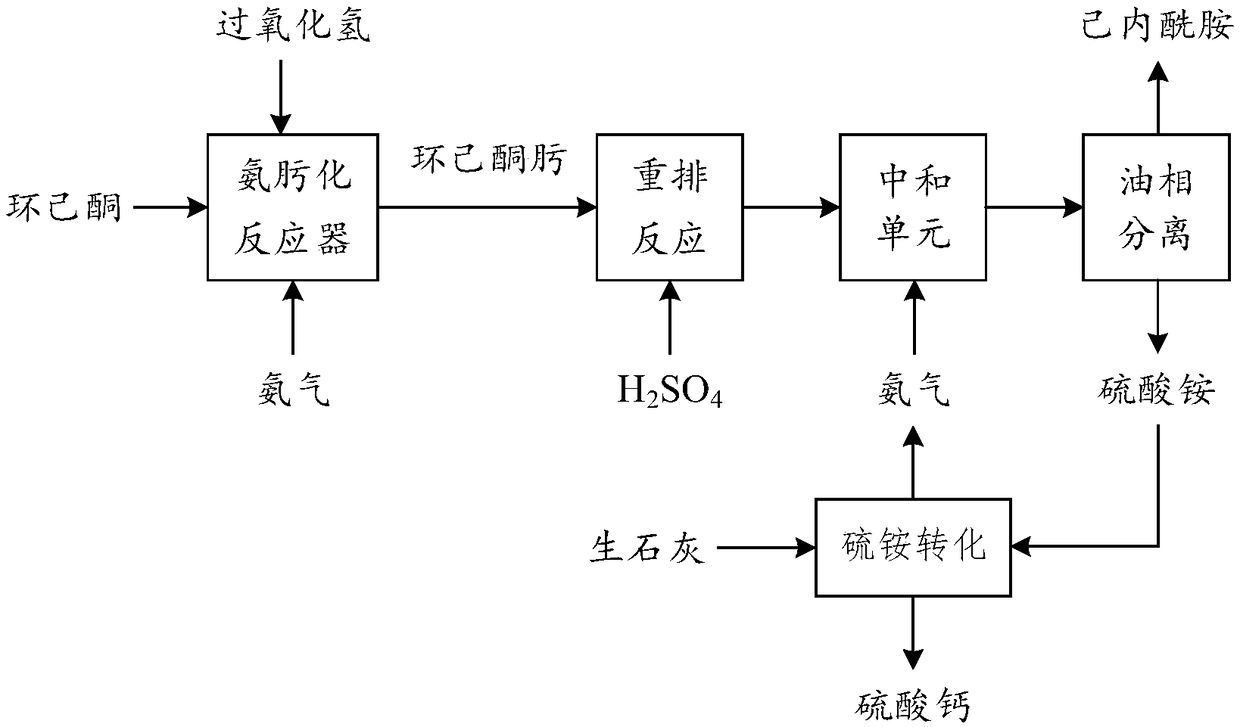
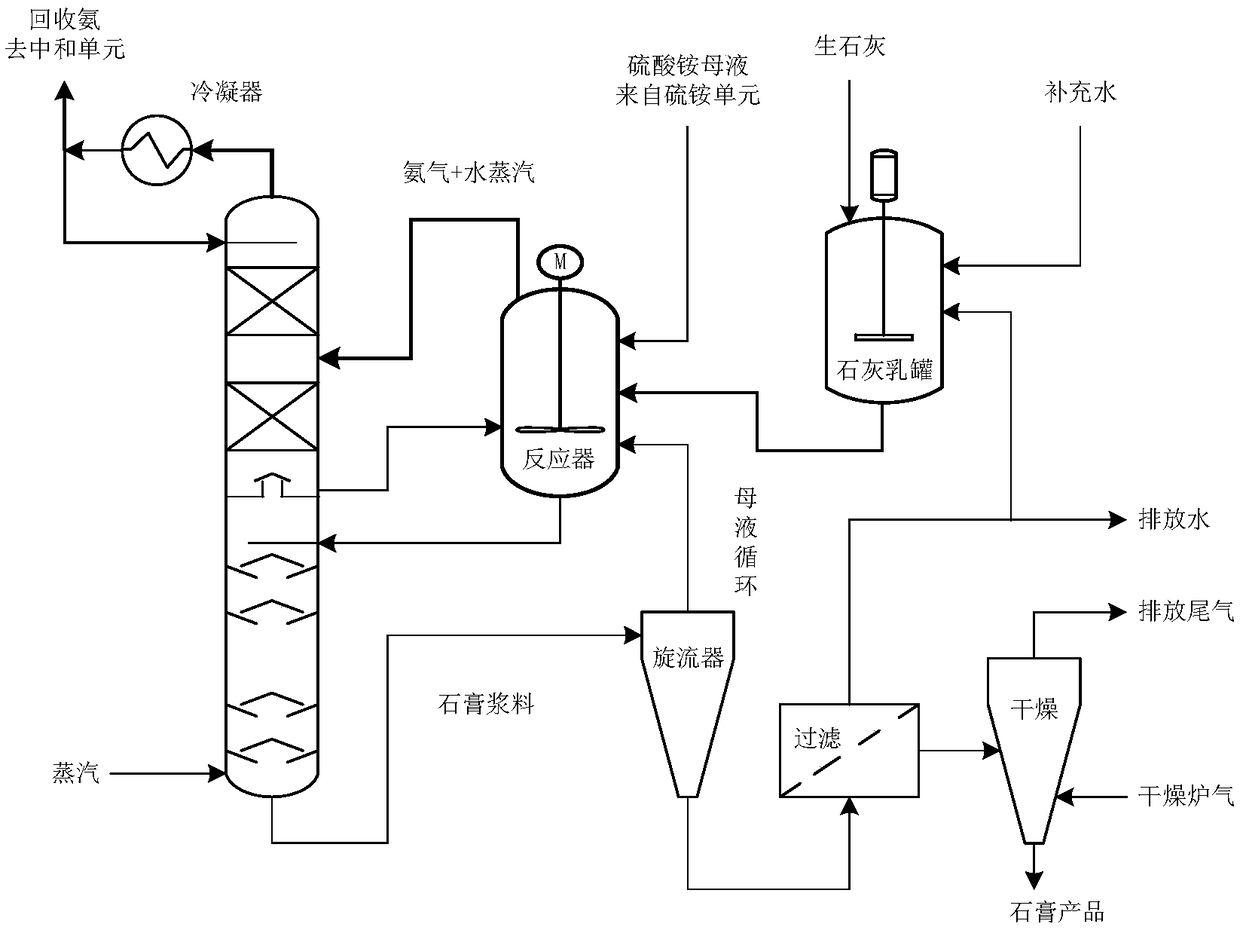
Low-ammonia-consumption caprolactam production process
A production process, a technology of caprolactam, applied to the preparation of lactam, calcium/strontium/barium sulfate, organic chemistry and other directions, can solve the problems of difficult industrial implementation, few recycling and replacement, complicated processes, etc. Economic benefits and the effect of reducing ammonia consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The crystallization law of the mixed reaction of ammonium sulfate and milk of lime was investigated experimentally in a 2L stirred tank. First in the stirred tank, the concentration of 900ml is 1.1mol / L of Ca(OH) 2 solution, mechanically stirred, and the outer jacket of the reactor was passed into heat conduction oil to heat and control the temperature, and the temperature was controlled at 100°C; then, 450ml concentration of 2.0mol / L (NH 4 ) 2 SO 4 The solution (21.2wt%) is poured into the reactor rapidly at one time, and the milk of lime remains excessive, and is uniformly mixed through mechanical stirring. After the reaction started, the pH value, the calcium ion concentration and the sulfate ion concentration of the measured solution were regularly sampled, and the crystal form and the particle size of the gypsum crystals generated by the sample analysis were analyzed simultaneously. During the reaction process, the nitrogen gas of 50ml / min is blown into the liqu...
Embodiment 2
[0041] The same as in Example 1, in a 2L stirred tank, the crystallization law of the mixed reaction of ammonium sulfate and milk of lime was experimentally investigated. The reaction temperature considered was also 100°C. The difference was the feeding method, and the milk of lime was added to sulfuric acid at one time under stirring conditions in ammonium solution. It is 2.0mol / L (NH 4 ) 2 SO 4 solution (21.2wt%), and then 900ml of Ca(OH) with a concentration of 1.1mol / L 2 The solution is poured into the reactor quickly at one time, and the milk of lime is kept in excess, and mechanical stirring is added to keep mixing evenly, and the nitrogen gas of 50ml / min is blown into the liquid phase simultaneously. After reacting for 60min, the ammonium ion concentration in the tail gas absorption bottle was sampled and analyzed, and the ammonium sulfate that added was used as a benchmark to calculate the ammonia recovery rate of 98.5wt%, indicating that ammonia was completely reco...
Embodiment 3
[0043] The same as in Example 1, in a 2L stirred tank, the crystallization law of the mixed reaction of ammonium sulfate and milk of lime was experimentally investigated. The difference was the reaction temperature and feeding method. The reaction temperature was 120 ° C. Under stirring conditions, the ammonium sulfate solution was pumped Continuously added to milk of lime. 900ml of Ca(OH) with a concentration of 1.1mol / L is charged into a 2L stirred tank earlier. 2 solution, and then 450ml concentration of 2.0mol / L (NH 4 ) 2 SO 4 Solution (21.2wt%) is continuously added in the reactor with the flow rate of 50ml / min, and ammonium sulfate solution has been added in 9 minutes, adds mechanical stirring and keeps mixing evenly, simultaneously the nitrogen gas of 50ml / min is blown into liquid phase. The jacket of the reactor was heated by heat conduction oil, and the temperature was controlled at 120°C. After reacting for 60min, the ammonium ion concentration in the tail gas ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com