Method for synthesizing cis-olefin by catalyzing decarboxylation coupling reaction of NHP ester and aryl-terminated alkyne with iridium
An aryl alkyne, decarboxylation coupling technology, applied in organic chemistry methods, chemical instruments and methods, condensation between hydrocarbons and non-hydrocarbons to produce hydrocarbons, etc., to achieve the effects of high yield, simple operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
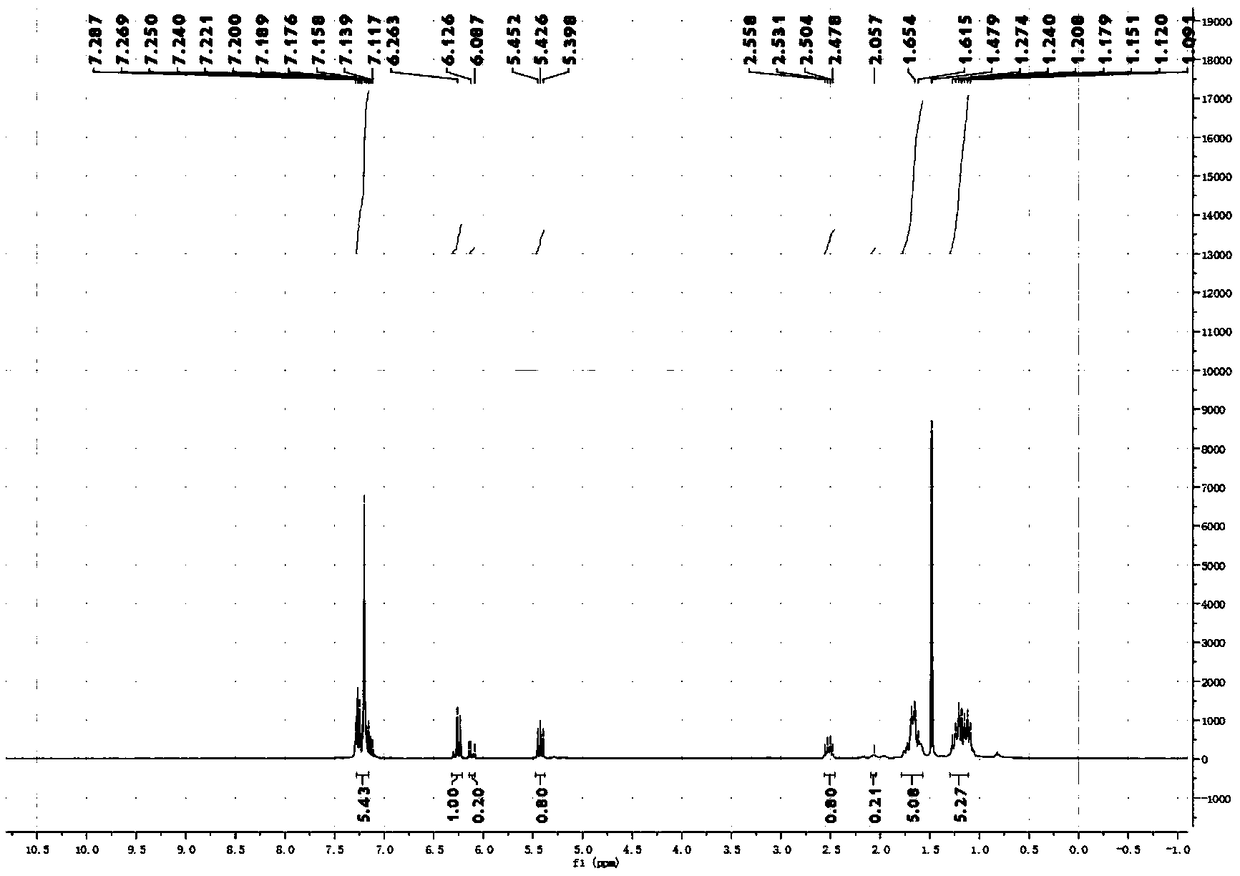
[0066] Synthesis and separation and purification of 1-(2-cyclohexylvinyl)-benzene:
[0067] Add the corresponding NHP ester (655.8mg, 0.4mmol, 6eq), Ir(ppy) 3 (2.6mg, 0.4mmol, 0.01eq); then add DIPEA (4.8mL; 68.7eq) and DMA (3.2mL) as solvents; degas with Ar balloon for 15min, then add phenylacetylene (44μL, 0.4mmol, 1eq), then seal the reaction bottle with a blue light of 36°C for light reaction for 18 hours; after the reaction is completed, extract the reaction mixture twice with 20mL EA, combine the organic phases and wash with saturated brine, then add anhydrous sodium sulfate to dry After the drying is completed, the organic solvent is spin-dried under reduced pressure to obtain a crude product; the crude product is separated by column separation using analytically pure PE as an eluent to obtain the final product; 1-(2-cyclohexylvinyl)benzene is a colorless oil Liquid, the productive rate is 71%, and wherein the ratio of cis-isomer and trans-isomer is 80:20 (cis-trans ra...
Embodiment 2
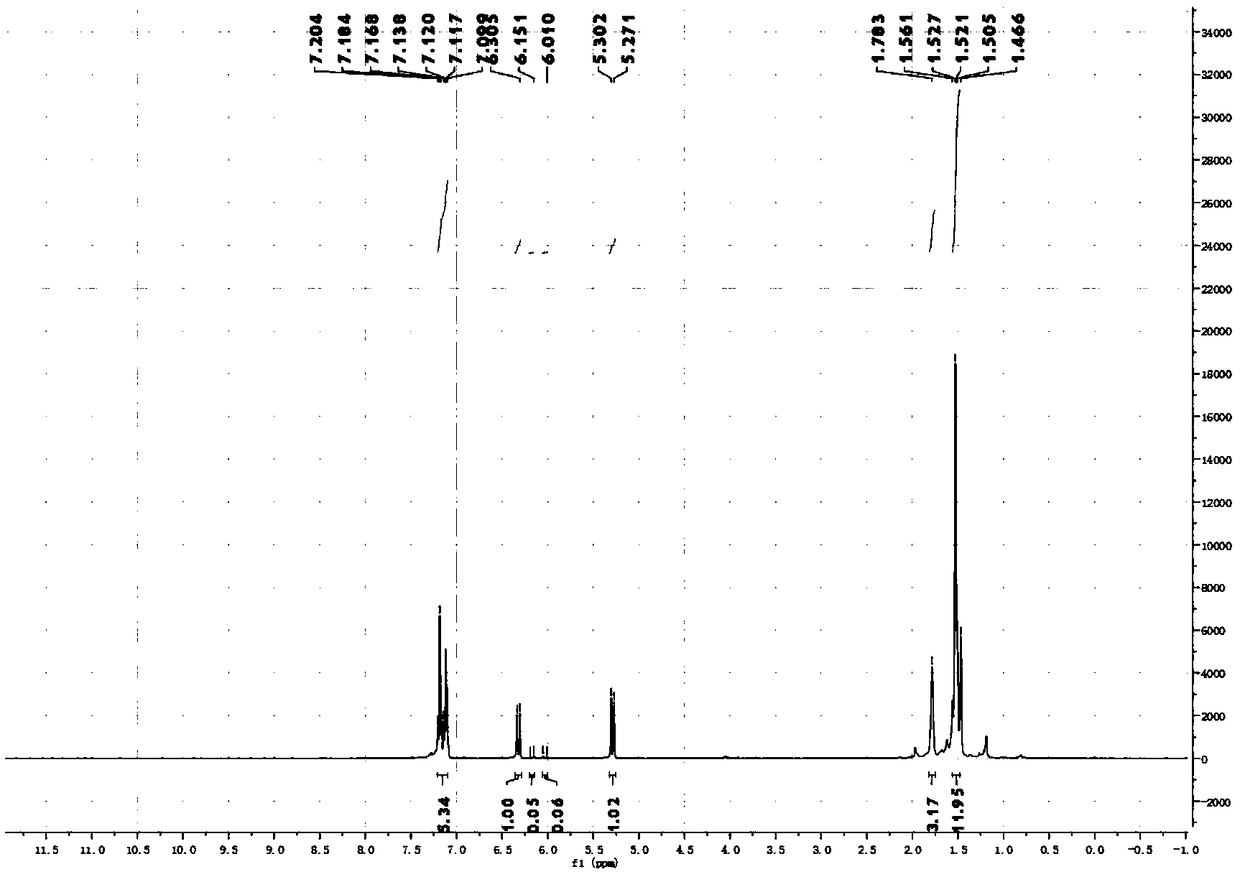
[0070] Synthesis, separation and purification of 1-(2-cyclohexylvinyl)-4-methyl carboxylate benzene: Add the corresponding NHP ester (655.8mg, 0.4mmol, 6eq) in sequence to a 10mL glass bottle equipped with a magnetic stirrer, Ir(ppy) 3 (2.6mg, 0.4mmol, 0.01eq); then DIPEA (4.8mL) and DMA (3.2mL) were added as solvents; a balloon filled with Ar was degassed for 15min, and then methyl 4-ethynylbenzoate (64mg, 0.4mmol, 1eq), then seal the reaction vial with a 36° blue light for light reaction for 18 hours; after the completion of the reaction, extract the reaction mixture twice with 20mL EA, combine the organic phases and wash with saturated saline, then add anhydrous Sodium sulfate drying; after drying, the organic solvent was spin-dried under reduced pressure to obtain a crude product; the crude product was separated by column using analytically pure PE as an eluent to obtain the final product as a colorless oily liquid with a yield of 75%. (The ratio of Z / E is 87:13)
[0071...
Embodiment 3
[0073] Synthesis, separation and purification of 1-(2-cyclohexylvinyl)-4-methylbenzene: Add the corresponding NHP ester (655.8mg, 0.4mmol, 6eq), Ir (ppy) 3 (2.6mg, 0.4mmol, 0.01eq); then DIPEA (4.8mL) and DMA (3.2mL) were added as solvents; a balloon filled with Ar was degassed for 15min, and then 4-methylphenylacetylene (50.8μL, 0.4 mmol, 1eq), then the reaction bottle was sealed with a blue light of 36°C for light reaction for 18 hours; after the reaction was completed, the reaction mixture was extracted twice with 20mL EA, the organic phases were combined and washed with saturated brine, and then anhydrous sulfuric acid was added Sodium drying; after drying, the organic solvent was spin-dried under reduced pressure to obtain a crude product; the crude product was separated by column using analytically pure PE as an eluent to obtain the final product as a colorless oily liquid with a yield of 68%. (The ratio of Z / E is 76:24)
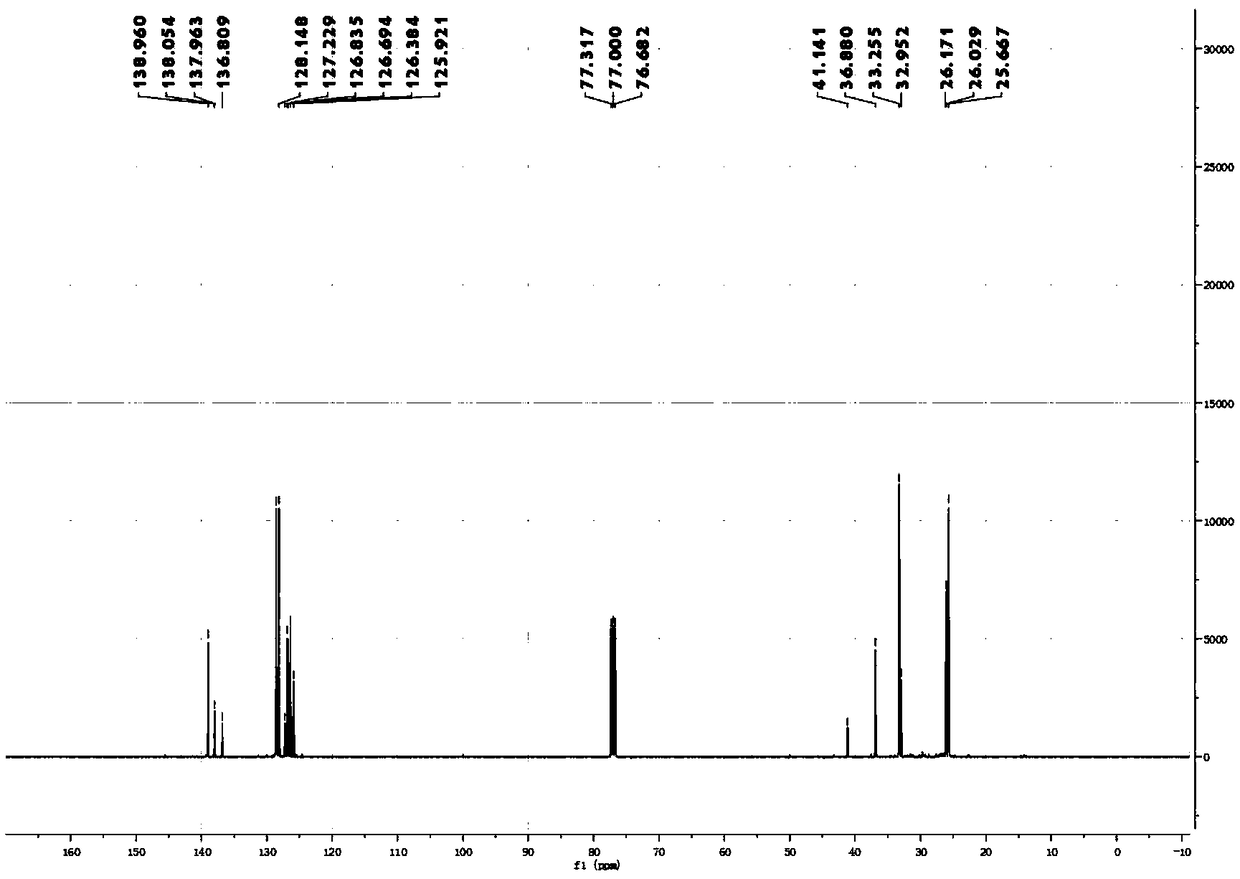
[0074] 1 H NMR (400MHz, CDCl 3): δ7.25-7.08(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com