Novel D-allulose 3-epimerase and application thereof
A technology of epimerase and psicose, applied in the field of genetic engineering, can solve the problems of unfavorable cost control and low thermal stability of D-psicose, and achieve low requirements for production conditions, high thermal stability, The effect of high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
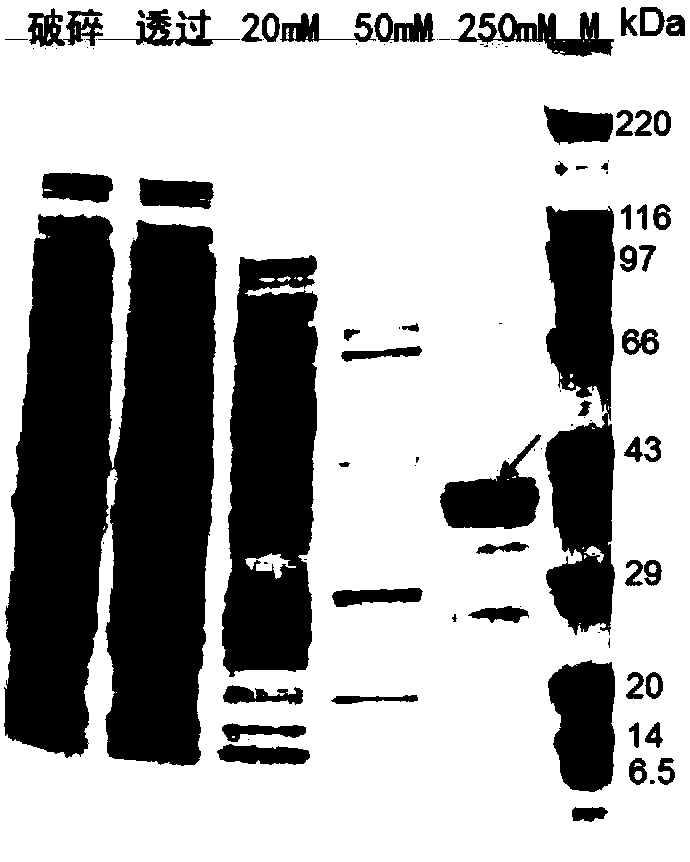
[0031] Example 1D-Expression of psicose 3-epimerase (MesDPE)
[0032]In the database, a potential thermostable sugar isomerase was searched and screened again, and a sugar phosphate isomerase from Mesoaciditoga lauensis was found. Its protein sequence is shown in SEQ ID NO: 1, and it is speculated that it has Ketose 3-epimerase activity. For high-efficiency expression in Escherichia coli, the coding sequence was optimized and screened through experiments to obtain the coding sequence shown in SEQ ID NO.2.
[0033] The construction of the Escherichia coli expression strain comprising SEQ ID NO.2 is as follows:
[0034] The coding sequence shown in SEQ ID NO.2 was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd., and a BamH I restriction site was added at its 5' end, and a histidine tag was added before the stop codon, and at the 3' end Add Pst I and Not I restriction sites. The sequence was ligated to pET 30a (+), pTac28 and pTST vectors (preserved in our laboratory) ...
Embodiment 2
[0038] The enzyme activity assay of embodiment 2 recombinant MesDPE
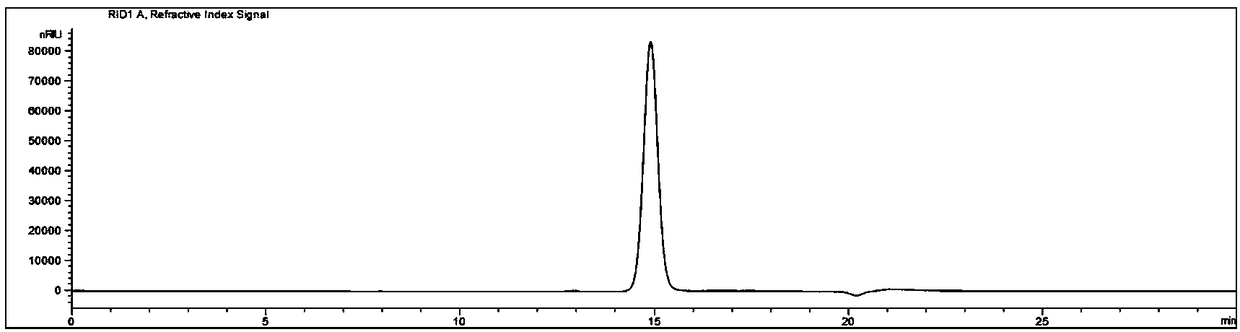
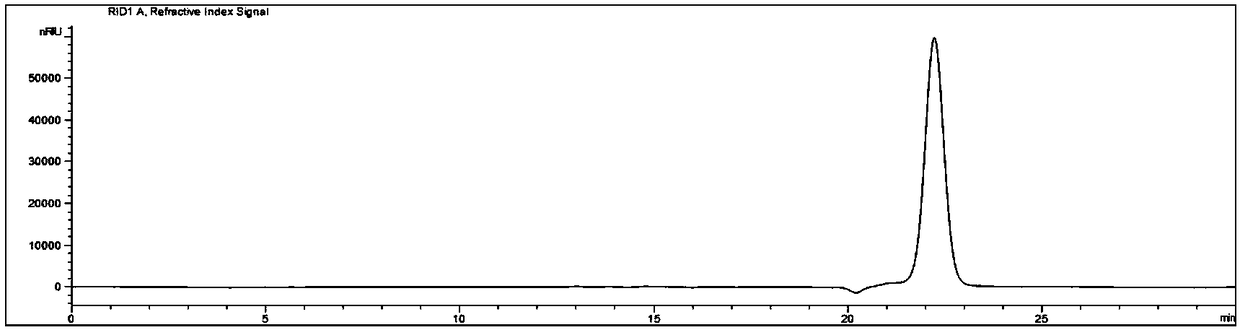
[0039] 1. The standard reaction system is as follows (1 mL): 143 μL purified enzyme MesDPE (final concentration: 0.02 mg / mL); 100 μL D-fructose solution (final concentration: 50 mg / mL); 10 μL Co 2+ (final concentration is 0.1mM CoCl 2 ); 690 μL Tris-HCl buffer (pH 8.0). The reaction conditions are: react in a water bath at 55°C for 20 minutes, and then treat in a boiling water bath for 5 minutes to inactivate the activity of the enzyme.
[0040] 2. The reaction system was filtered with a 0.45 μm microporous membrane, and the filtrate was used for high performance liquid phase analysis. The high performance liquid chromatography conditions are as follows: Agilent 1260HPLC and G1362A RID detector; analytical column: Waters Sugar-Pak I, 6.5×300mmcolumn; mobile phase: water; flow rate: 0.4mL / min; column temperature: 80°C; detector: RID , the detector temperature was 55°C; the sample volume was 20 μL.
[0041...
Embodiment 3
[0043] Embodiment 3 Identification of recombinant MesDPE enzymatic properties
[0044] 1. Determination of metal ion dependence: The reaction system was configured according to the final concentration of substrate fructose of 50 mg / mL, the final concentration of metal ions of 1 mM, and the final concentration of enzyme of 0.02 mg / mL; carried out according to the reaction conditions in Example 2. like Figure 5 As shown, the activity of MesDPE mainly depends on Co 2+ , followed by Mn 2+ .
[0045] 2. The influence of pH on enzyme activity: according to the standard reaction among the embodiment 2, take 50mg / mL fructose as substrate, Co 2+ The final concentration is 0.1mM, and the following buffers are used respectively: 50mM sodium dihydrogen phosphate-sodium hydroxide buffer solution of pH5-7; 50mM Tris-HCl buffer solution of pH7.5-9.5; according to the reaction conditions in Example 2 . The results show( Image 6 ), MesDPE had the highest activity at pH 6.0; as the pH v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com