A kind of polycarbazole loaded nano-palladium material and its preparation method and application
A nano-palladium, polycarbazole technology, applied in the field of catalytic chemistry, achieves the effects of high conversion efficiency, green and mild reaction conditions, and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Pd(OAc) 2 (11.2 mg), carbazole polymer (120 mg) were added to a 100 ml three-necked round bottom flask containing a magnetic stirrer, stirred at room temperature for 30 minutes, and then NaBH was added to it through a syringe 4 (0.05 M, 5.0 mL) aqueous solution, and reacted at room temperature for 4 hours; after the reaction, the solid was separated by centrifugation, washed with water, ethanol and ether in turn, and then vacuum-dried to obtain the corresponding polycarbazole-loaded nano-palladium material (Pd / P3,5-diCzPy).
[0052] ICP analysis indicated that the palladium loading was 1.37% wt.
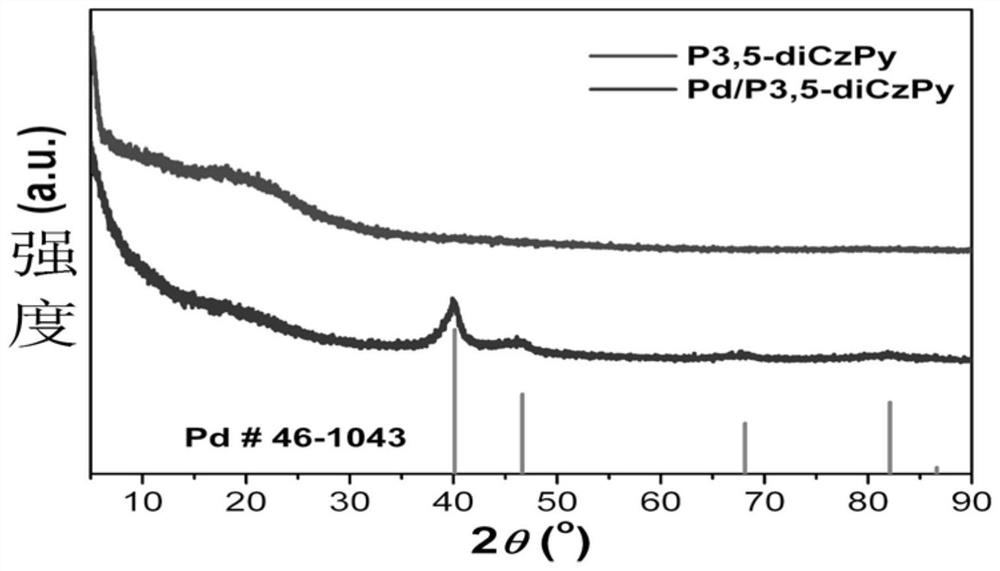
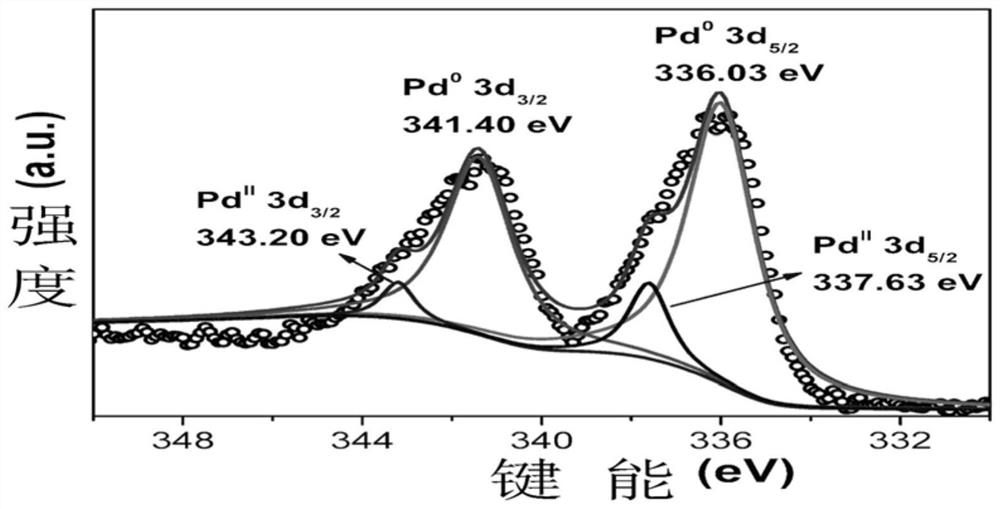
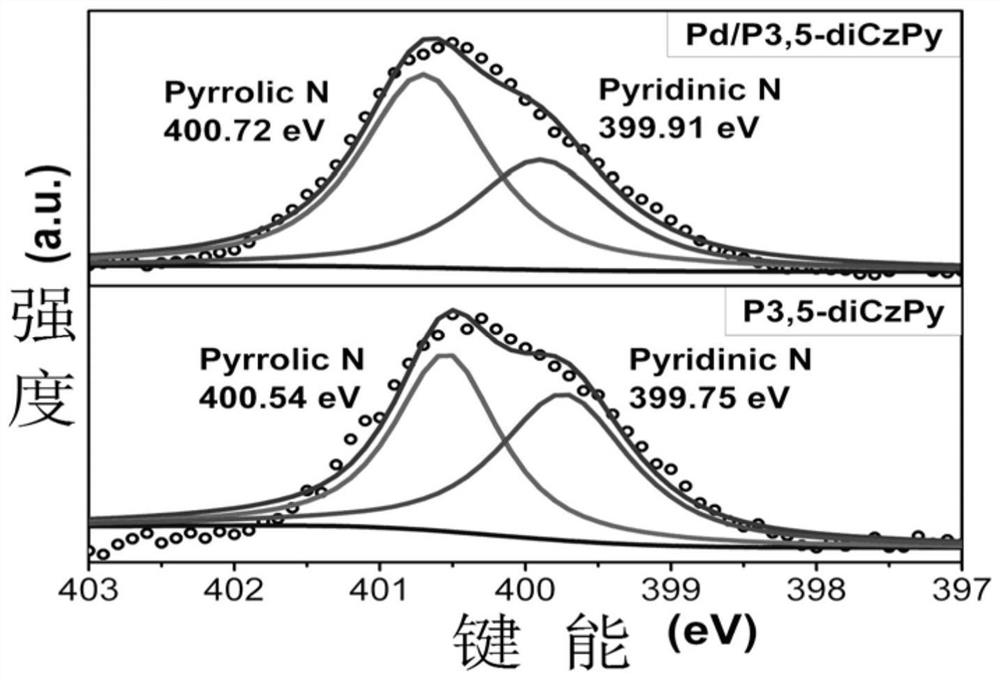
[0053] Figure 1 to Figure 6 Followed by the powder XRD diffraction pattern, photoelectron energy spectrum, transmission electron microscope and particle size distribution, high-resolution transmission electron microscope and element distribution diagram of the palladium nanomaterial supported by the carbazole polymer of the present invention; the powder XRD diffraction pe...
Embodiment 2
[0055]
[0056] Add 4'-bromoacetophenone (40 mg), phenylboronic acid (37 mg), polycarbazole-supported nano-palladium material (8 mg), and potassium phosphate (64 mg) into a 10 ml quartz reaction tube equipped with a magnetic stirring bar 5 ml of deionized water was added, and the liquid nitrogen freezing-pumping-nitrogen-filling-thawing was repeated three times, and then under the irradiation of blue LED, the airtight room temperature was reacted for 12 h; after the reaction, the catalyst was removed by filtration, and the catalyst was washed with ethyl acetate. , ethyl acetate extracted the filtrate, combined the organic phases, dried, filtered, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain 4-biphenylethanone (94% yield); using palladium carbon catalyst, the yield was 12 %, adopting palladium chloride yield is 29%.
[0057] The NMR data of the resulting product are as follows:
[0058] 1 H NMR (600 MHz, CDCl 3 ) δ 8.03 (...
Embodiment 3
[0064]
[0065] Add 4'-bromoacetophenone (40 mg), 4-methylphenylboronic acid (41 mg), polycarbazole-loaded nano-palladium material (8 mg), and potassium phosphate (64 mg) to a magnetic stirring bar 10 5 ml of deionized water was added to a quartz reaction tube, and the liquid nitrogen freezing-pumping-nitrogen filling-thawing was repeated three times, and then under the irradiation of a blue LED, the reaction was performed at room temperature for 12 h; after the reaction, the catalyst was removed by filtration, and the acetic acid Wash the catalyst with ethyl acetate, extract the filtrate with ethyl acetate, combine the organic phases, dry, filter, concentrate under reduced pressure, and purify by silica gel column chromatography to obtain 4'-methyl-4-biphenylphenone (98% yield) , if adopting diisopropylamine as the base, the yield is 16%.
[0066] The NMR data of the resulting product are as follows:
[0067] 1 H NMR (400 MHz, CDCl 3 ) δ 7.97 (d, J = 8.1 Hz, 2H), 7.62...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com