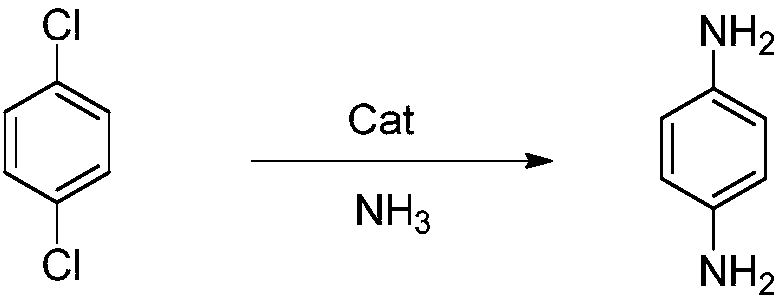
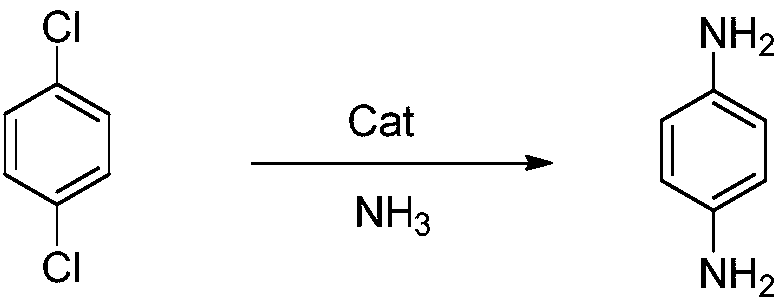
Preparation method of p-phenylenediamine
A technology for p-phenylenediamine and p-dichlorobenzene, which is applied in the field of preparation of p-phenylenediamine, can solve the problems of low atom utilization rate, seriousness, long reaction time, etc. The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 1L autoclave, add 117.72g p-dichlorobenzene (99.9%, 0.8mol), 437.14g ammonia water (35%, 9.0mol), 7.93g cuprous chloride (99.9%, 0.08mol), 28.32g Mannich base ligand L3 (0.08mol), after the feeding is completed, the temperature is raised to 130°C, the stirring speed is 600rpm, the heat preservation reaction is 6hr, and the pressure is 2.2Mpa. After the reaction, the ammonia is recovered by cooling down and releasing the pressure, and 77.15g of p-benzene Diamine, content 99.9%, yield 89.2%.
Embodiment 2
[0024] In 1L autoclave, add 58.86g p-dichlorobenzene (99.9%, 0.4mol), 355.56g ammonia water (45%, 8.0mol), 0.76g cuprous iodide (99.9%, 0.004mol), 4.20g Mannich base ligand L1 (0.008mol), after the feeding is completed, the temperature is raised to 150°C, the stirring speed is 600rpm, the heat preservation reaction is 10hr, and the pressure is 4.2MPa. After the reaction is completed, the temperature is lowered and the pressure is released to recover ammonia. Diamine, content 99.9%, yield 90.6%.
Embodiment 3
[0026] Add 88.30g p-dichlorobenzene (99.9%, 0.6mol), 353.80g ammonia water (40%, 7.2mol), 0.30g cuprous chloride (99.9%, 0.003mol), 3.93g Mannich base ligand in 1L autoclave L2 (0.009mol), after the feeding is completed, the temperature is raised to 150°C, the stirring speed is 600rpm, the heat preservation reaction is 24hr, and the pressure is 3.2MPa. After the reaction, the ammonia is recovered by cooling down and releasing the pressure. Diamine, content 99.9%, yield 86.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com