Catalyst of imide synthesized by aromatic nitro compound and benzaldehyde or furfural and derivative thereof as well as preparation method and application
A technology of aromatic nitro group and derivatives is used in catalysts, preparation methods and application fields for synthesizing imines from aromatic nitro compounds, benzaldehyde or furfural and derivatives thereof, and can solve the problem of increasing reaction costs, hidden dangers of operator safety, High reaction temperature and other problems, to achieve the effect of low production cost, low equipment investment and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
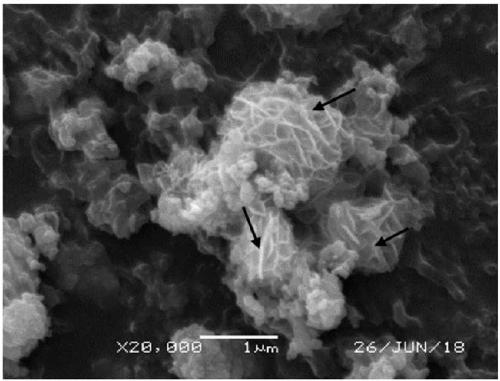
[0046] CoS 2 @MoS 2 -220-0.25 preparation: get 750mg ammonium heptamolybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O), 252.8mg cobalt chloride (CoCl 2 ·6H 2 0), add 30mL ethylene glycol and 30mL deionized water to make a mixed solution, the total concentration of the mixed solution is 0.089mol / L, and the mixed solution is constantly stirred at room temperature. Add 510mg of sulfur powder to the mixed solution obtained above and keep stirring for 0.5h, make it fully mixed, then add 6mL of hydrazine hydrate, and stir vigorously for 1h. The resulting mixed solution was raised to 220° C. at a heating rate of 5° C. / min and left to react for 6 h. The obtained catalyst was washed with deionized water and ethanol three times respectively, and then vacuum-dried at 40° C. for 24 h.
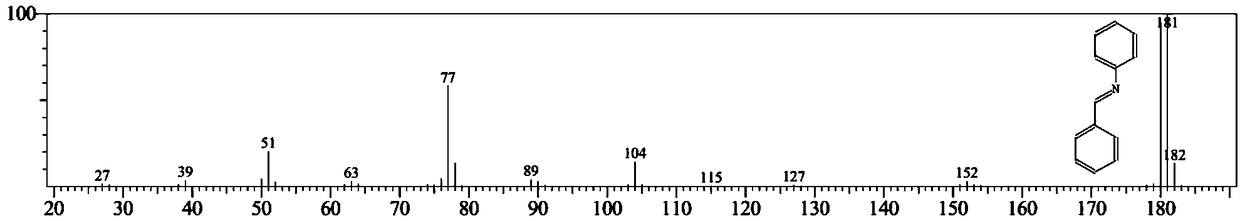
[0047]In a 50mL autoclave reactor, add nitrobenzene (123mg, 1mmol), benzaldehyde (138mg, 1.3mmol), 80mg of catalyst, ethanol (10mL) and a stirring magnet, and replace the reaction system with hydrogen for 5 ti...
experiment example 2
[0049] CoS 2 @MoS 2 -160-1 preparation: get 750mg ammonium heptamolybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O), 1011.2mg cobalt chloride (CoCl 2 ·6H 2 0), add 10mL ethylene glycol and 50mL deionized water to make a mixed solution, the total concentration of the mixed solution is 0.14mol / L, and the mixed solution is constantly stirred at room temperature. Add 816 mg of sulfur powder to the mixed solution obtained above and keep stirring for 1 h, make it fully mixed, then add 10 mL of hydrazine hydrate, and stir vigorously for 1 h. The resulting mixed solution was raised to 160° C. at a heating rate of 5° C. / min and left to react for 24 hours. The obtained catalyst was washed with deionized water and ethanol three times respectively, and then vacuum-dried at 60° C. for 6 h.
[0050] In a 50mL autoclave reactor, add nitrobenzene (123mg, 1mmol), p-chlorobenzaldehyde (183mg, 1.3mmol), 80mg of catalyst, ethanol (10mL) and a stirring magnet, and replace the reaction system with hydr...
experiment example 3
[0052] CoS 2 @MoS 2 -180-0.5 preparation: get 750mg ammonium heptamolybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O), 505.6mg cobalt chloride (CoCl 2 ·6H 2 0), add 50mL ethylene glycol and 10mL deionized water to make a mixed solution, the total concentration of the mixed solution is 0.106mol / L, and the mixed solution is constantly stirred at room temperature. Add 612 mg of sulfur powder to the mixed solution obtained above and keep stirring for 1 h, make it fully mixed, then add 8 mL of hydrazine hydrate, and stir vigorously for 2 h. The resulting mixed solution was raised to 180° C. at a heating rate of 10° C. / min and left to react for 12 hours. The obtained catalyst was washed with deionized water and ethanol three times respectively, and then vacuum-dried at 80° C. for 12 h.
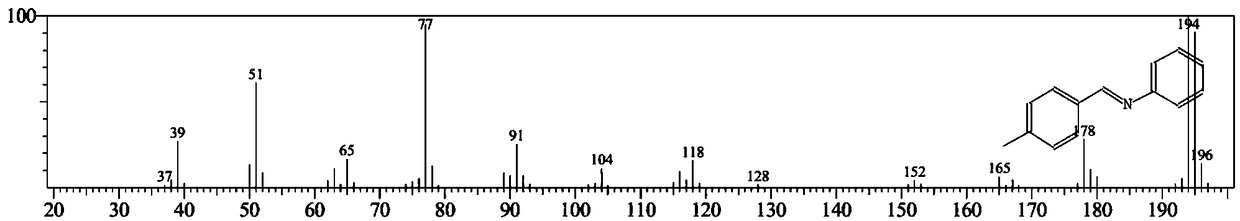
[0053] In a 50mL autoclave reactor, add nitrobenzene (123mg, 1mmol), p-tolualdehyde (240mg, 2mmol), 80mg of catalyst, ethanol (10mL) and a stirring magnet, and replace the reaction system with hydrogen ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com