Novel heterocyclic compounds and medicinal application of same as anti-influenza virus inhibitors
A kind of technology of heterocyclic compound and medicinal salt, applied in the application field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
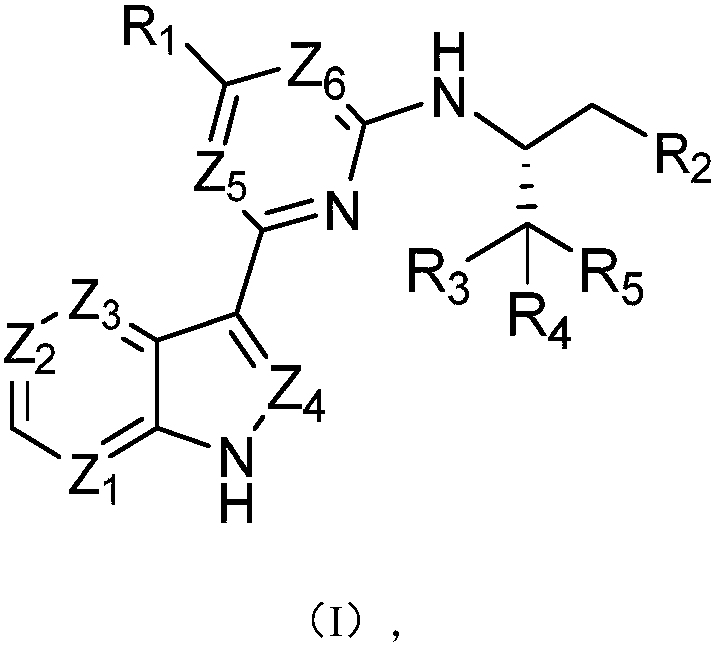
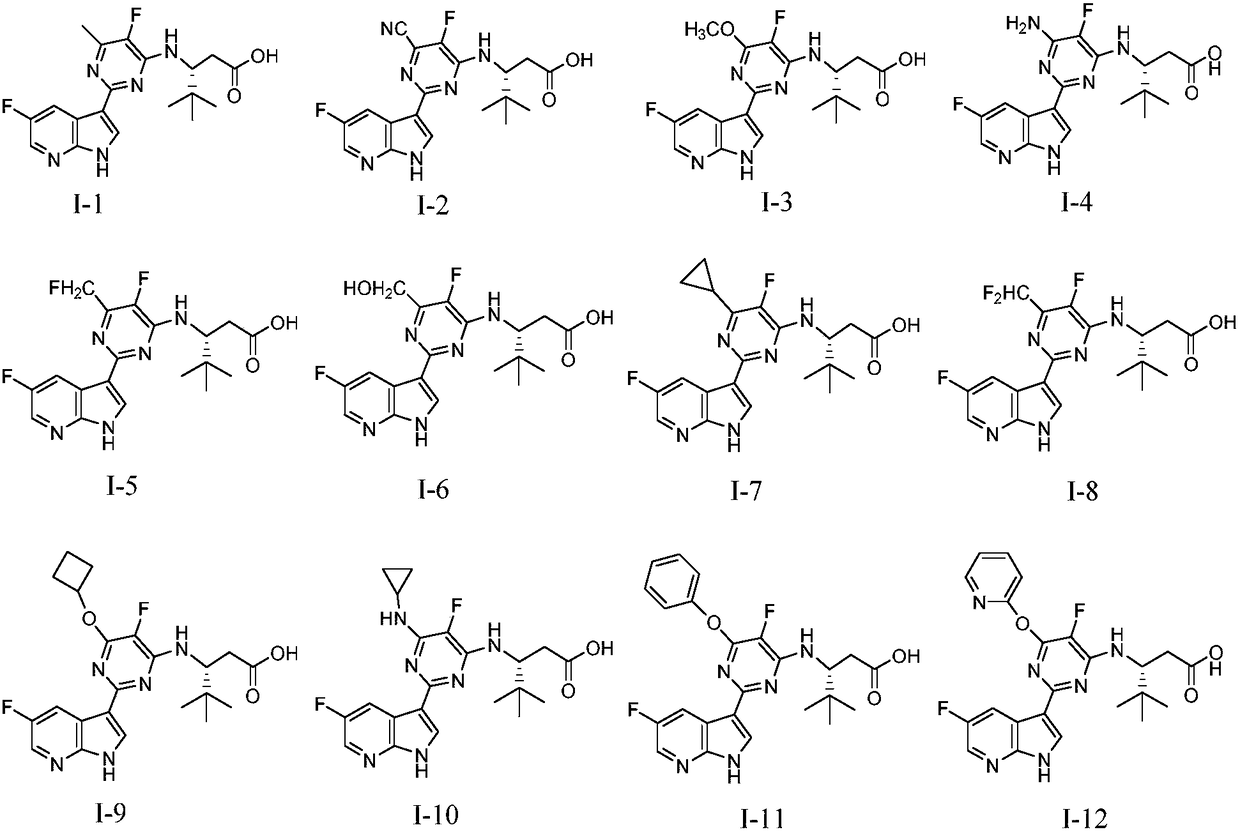
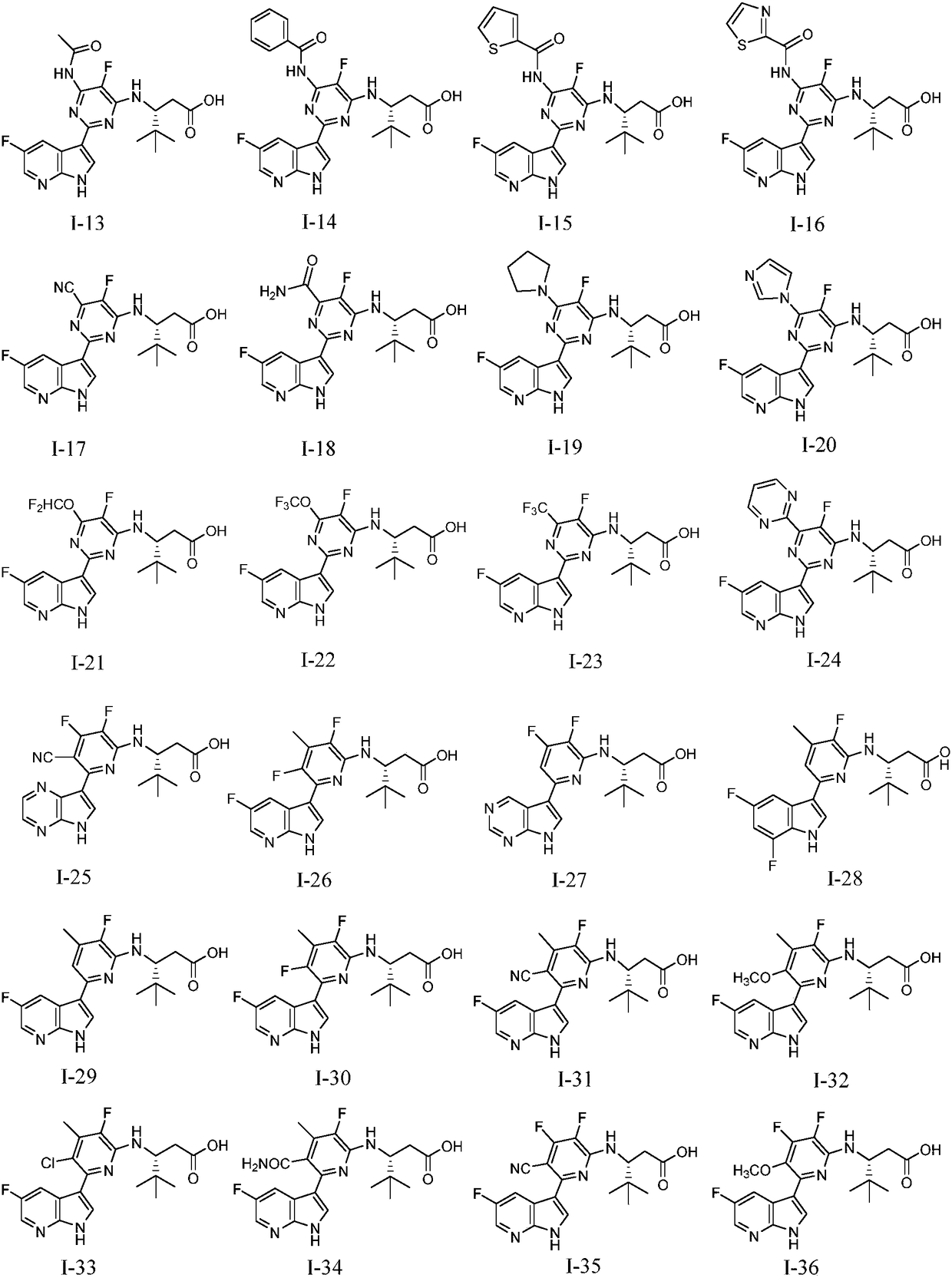
[0088] Example 1: Preparation of (R)-3-((5-fluoro-2-(5-fluoro-1H-pyrrolo[2,3-b]pyridin-3-yl)-6-methylpyrimidine-4- base)amino)-4,4-dimethylpentanoic acid (I-1)
[0089]
[0090] Preparation of compound 2: N-bromosuccinimide (NBS, 5.29g, 29.7mmol) was added to a solution of compound 1 (4.50g, 33mmol) in dichloromethane (100mL) and reacted at room temperature for 19 hours, After the reaction was completed, saturated sodium bisulfite solution (200ml) was added, the layers were separated, the organic layer was washed with 20% sodium hydroxide solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated in vacuo to obtain 4.62g of crude product.
[0091] Preparation of Compound 3: Compound 2 (4.40 g, 20.4 mmol) was dissolved in 30 mL of dry DMF, sodium hydrogen (1.30 g, 32.6 mol) was added and stirred for 30 minutes. Then p-toluenesulfonyl chloride (TsCl, 5.78 g, 30.6 mmol) was added and reacted for 4 hours. After the reaction was completed, it wa...
Embodiment 2
[0099] Example 2: Preparation of (R)-3-((5-fluoro-2-(5-fluoro-1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)- 4,4-Dimethyl-N-(methylsulfonyl)pentanamide (I-65)
[0100]
[0101] Preparation of Compound 11: Compound 4 (1.40g, 3.36mmol), 2-chloro-4-thiomethyl-5-fluoropyrimidine (0.50g, 2.80mmol), Pd2(dba)3 (0.26g, 0.28mmol), X-phos (0.67g, 1.4mmol) and sodium carbonate (0.89g, 8.40mmol) were dissolved in 25mL of ethylene glycol dimethyl ether and 5mL of water, nitrogen bubbling for 30 minutes to remove the oxygen in the system, heated to reflux for 19 hours, cooled After reaching room temperature, the reaction solution was poured into 100 mL of water, filtered, the filter cake was washed with water, and then recrystallized with acetonitrile to obtain 1.10 g of the product. Yield: 75.7%.
[0102] Preparation of Compound 12: At 0°C, m-chloroperoxybenzoic acid (MCPBA, 85%, 0.71g, 3.48mmol) was added in batches to a solution of Compound 11 (0.60g, 1.39mmol) in dichlorometh...
Embodiment 3
[0108] Example 3. In vitro biological activity studies and cytotoxicity studies
[0109] Compounds to be tested: compounds of the present invention: compound I-1, compound I-65; reference compound: VX-787.
[0110] Test method for in vitro biological activity research: MDCK cells were seeded into 384-well cell culture plates at a density of 2,000 cells per well, and then placed at 37°C, 5% CO 2 Incubate overnight in the incubator. The next day, the compounds were diluted and added to the cell wells (3-fold dilution, 8 test concentration points), and then the influenza virus A / PR / 8 / 34 (H1N1) strain was added to the cell culture wells at 2*TCID90 per well , the final concentration of DMSO in the medium was 0.5%. Place the cell plate at 37°C, 5% CO 2 Cultured in the incubator for 5 days. After 5 days of culture, the cell viability was detected using the cell viability detection kit CCK8. The original data was analyzed by nonlinear fitting of the inhibitory rate and cytotoxic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com