An Electrochemical Method for Online Analysis of Nitro Reduction Reaction
A technology of electrochemistry and nitro compounds, applied in the direction of electrochemical variables of materials, etc., can solve the problems of high detection consumption, complicated operation, long time consumption, etc., and achieve the effect of simple detection device, good electrochemical response, and feasibility of solving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
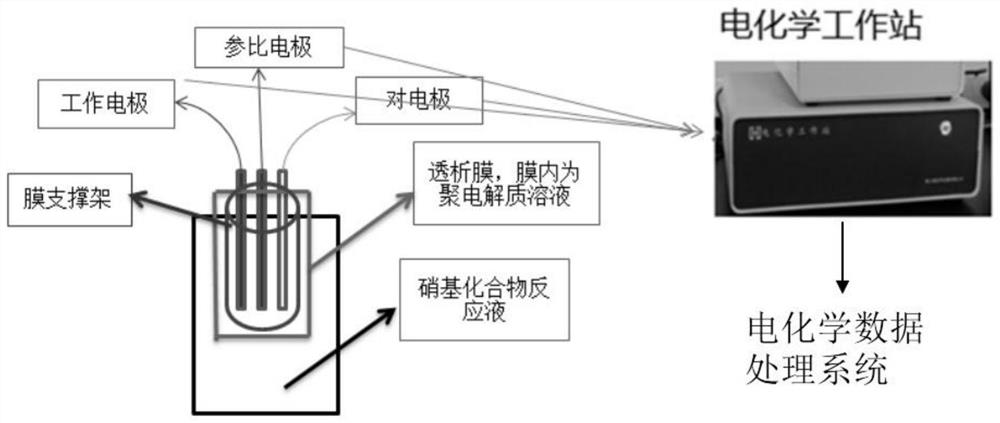
[0038](1) Add 510 mg of polydiallyl ammonium chloride to 50 mL of DMF and mix to prepare a polyelectrolyte solution. Take 3 to 5 mL of the polyelectrolyte solution and place it in a dialysis membrane device, wherein the molecular weight cut-off of the dialysis membrane is 3500;
[0039] (2) The surface of the working electrode (platinum disk microelectrode) needs to be cleaned before use. Use 0.05μm a-AL 2 o 3 Powder polished, then rinsed with ultrapure water; wipe dry for use.
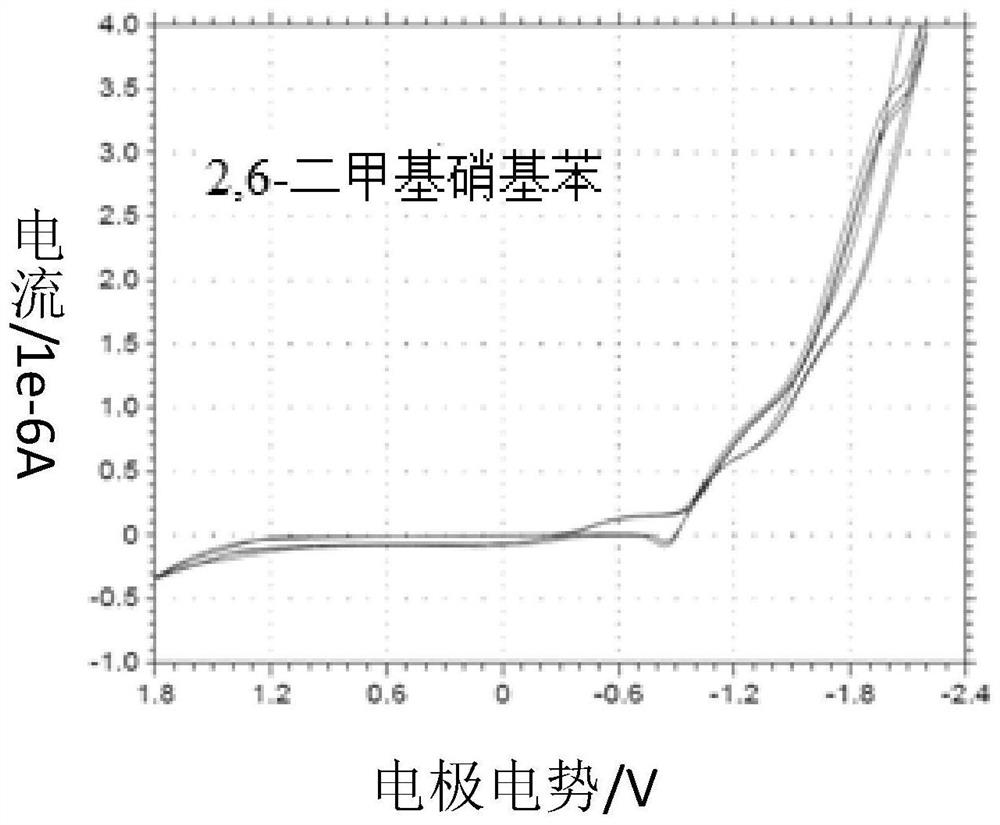
[0040] (3) Place the working electrode (platinum disk microelectrode), counter electrode (platinum wire) and reference electrode (silver / silver chloride electrode) in the dialysis membrane device in (1), and then mix with 2,6-di The reaction solution of methylnitrobenzene (solvent is DMF) is connected to the electrochemical system circuit, and the working voltage is set in the range of -2.0 ~ 1.8v by electrochemical cyclic voltammetry, and the 2,6-dimethylnitrobenzene The reaction solution is analyz...
Embodiment 2
[0042] (1) Add 490mg of polystyrene sulfonic acid to 50ml of methanol and mix to prepare a polyelectrolyte solution. Get 3 to 5ml of the polyelectrolyte solution and place it in a dialysis membrane device, wherein the molecular weight cut-off of the dialysis membrane is 7000;
[0043] (2) The surface of the working electrode (platinum disk microelectrode) needs to be cleaned before use. Use 0.05μm a-AL 2 o 3 Powder polished, then rinsed with ultrapure water; wipe dry for use.
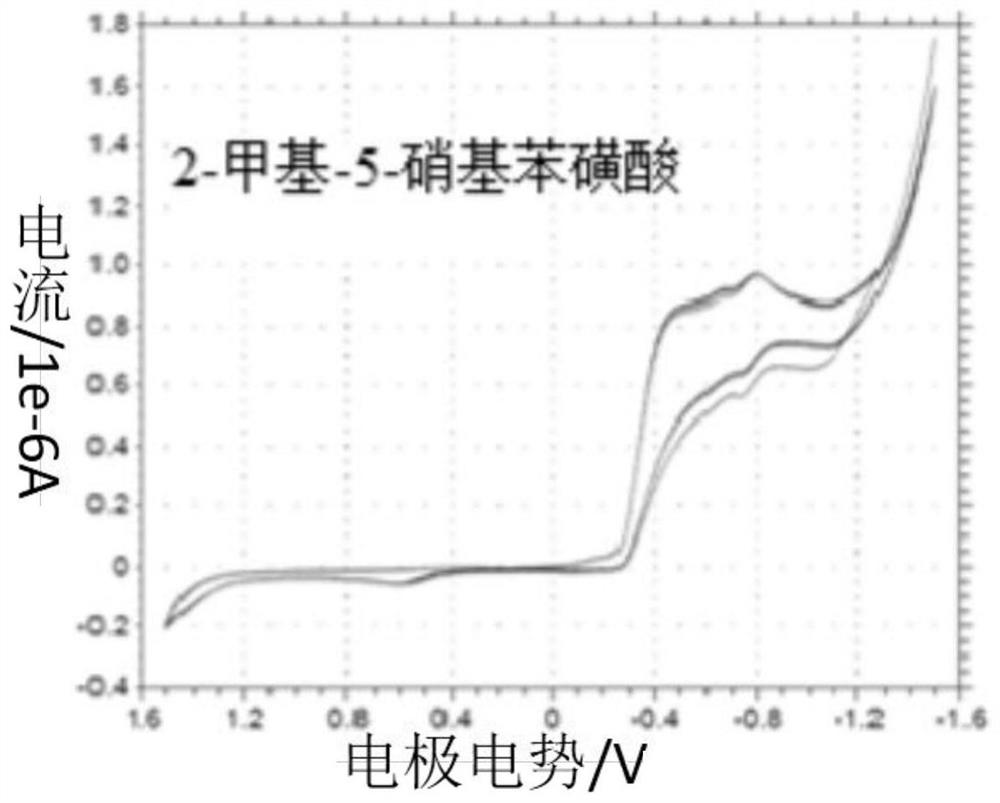
[0044] (3) Place the working electrode (platinum disk microelectrode), counter electrode (tungsten wire) and reference electrode (saturated calomel electrode) in the dialysis membrane device in (1), and then mix with 2-methyl-5- The nitrobenzenesulfonic acid reaction solution (the solvent is methanol) is connected to the electrochemical system circuit, and the operating voltage is set at -2.0 to 2.0v by electrochemical cyclic voltammetry, and the p-2-methyl-5-nitrobenzene The sulfonic acid reaction so...
Embodiment 3
[0046] (1) Add 512mg polydiallyldimethylammonium chloride to 50ml DMAC and mix to prepare a polyelectrolyte solution, take 3-5ml polyelectrolyte solution and place it in a dialysis membrane device, wherein the molecular weight cut-off of the dialysis membrane is 50000 ;
[0047] (2) The surface of the working electrode (platinum disk microelectrode) needs to be cleaned before use. Use 0.05μm a-AL 2 o 3 Powder polished, then rinsed with ultrapure water; wipe dry for use.
[0048] (3) Place the working electrode (platinum disk microelectrode), counter electrode (tungsten wire) and reference electrode (silver / silver chloride electrode) in the dialysis membrane device in (1), and then mix with 4-chloro-3 -The nitroanisole reaction solution (the solvent is DMAC) is connected to the electrochemical system circuit, using electrochemical cyclic voltammetry, the working voltage is set at -2.0 ~ 1.8v range, the 4-chloro-3-nitrobenzene The methyl ether reaction solution was analyzed a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com