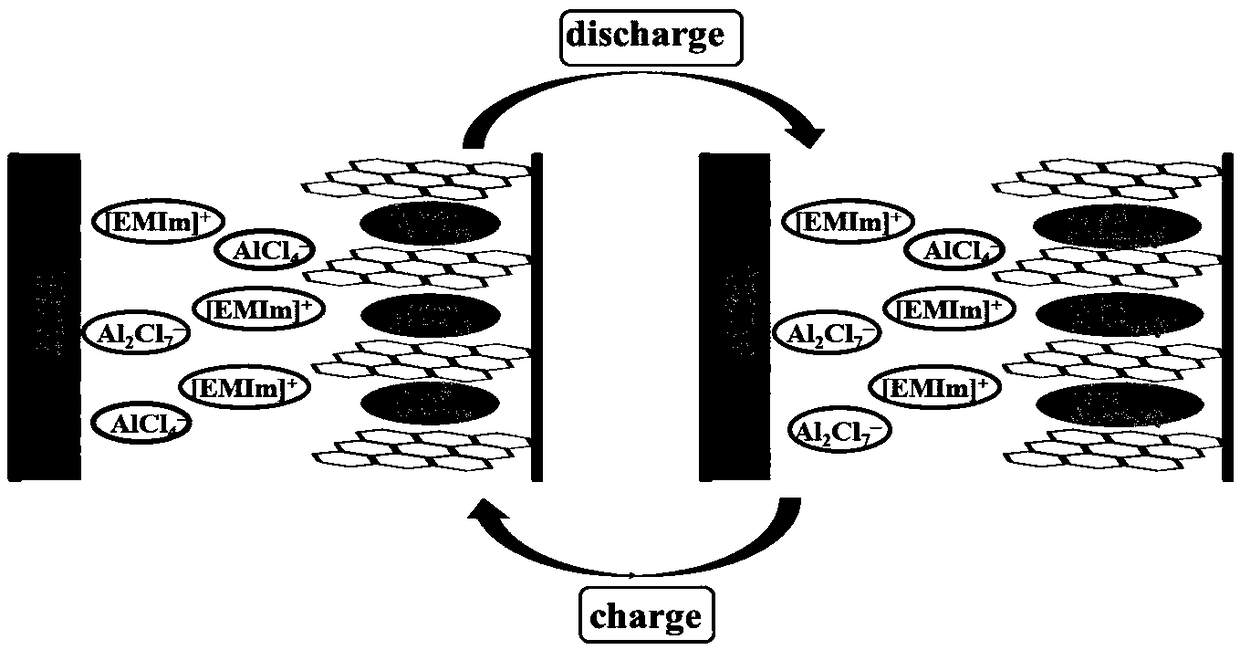
Novel aluminum-chlorine mixed ion battery
A technology of mixed ions and aluminum chloride, which is applied in the field of electrochemical energy storage, can solve the problems that hinder the popularization and application of aluminum ion batteries, the lack of specific energy and long cycle life of positive electrode materials in aluminum ion batteries, difficult insertion/extraction, etc., to achieve extended activity Potential, enhanced rate performance, and good electrochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0031] With metal aluminum as the negative electrode, AlCl 3 -[EMIm]Cl is the electrolyte, with CuCl 2 -GIC is the positive electrode material, assembled into an aluminum-chloride mixed ion battery.
[0032] The preparation method of the electrolyte is: weigh 7.31g [EMIm]Cl in the glove box, then weigh 8.64g AlCl 3 , the AlCl 3 Slowly add it to [EMIm]Cl until the ionic liquid turns transparent, which is the prepared electrolyte.
[0033] CuCl 2 -The preparation method of GIC cathode material: weigh 0.12g of natural flake graphite and 1.11g of CuCl in the glove box 2 , wherein the molar ratio of graphite to chloride is 2:3, put it into a quartz tube and vacuumize it for 5 minutes and then seal it. Put the sealed quartz test tube into the muffle furnace, raise the temperature to 380°C at 5°C / min, keep the temperature for 2h, and cool down to room temperature naturally. After the sample was taken out, it was washed with deionized water until it had no color, and dried in a ...
specific Embodiment 2
[0036] With metal aluminum as the negative electrode, AlCl 3 and [EMIm]Cl as the electrolyte, with FeCl 3 -GIC is the positive electrode material, assembled into an aluminum-chloride mixed ion battery. The molar ratio of the electrolyte [EMIm]Cl:AlCl 3 =1:1.5. The preparation method is as in Example 1.
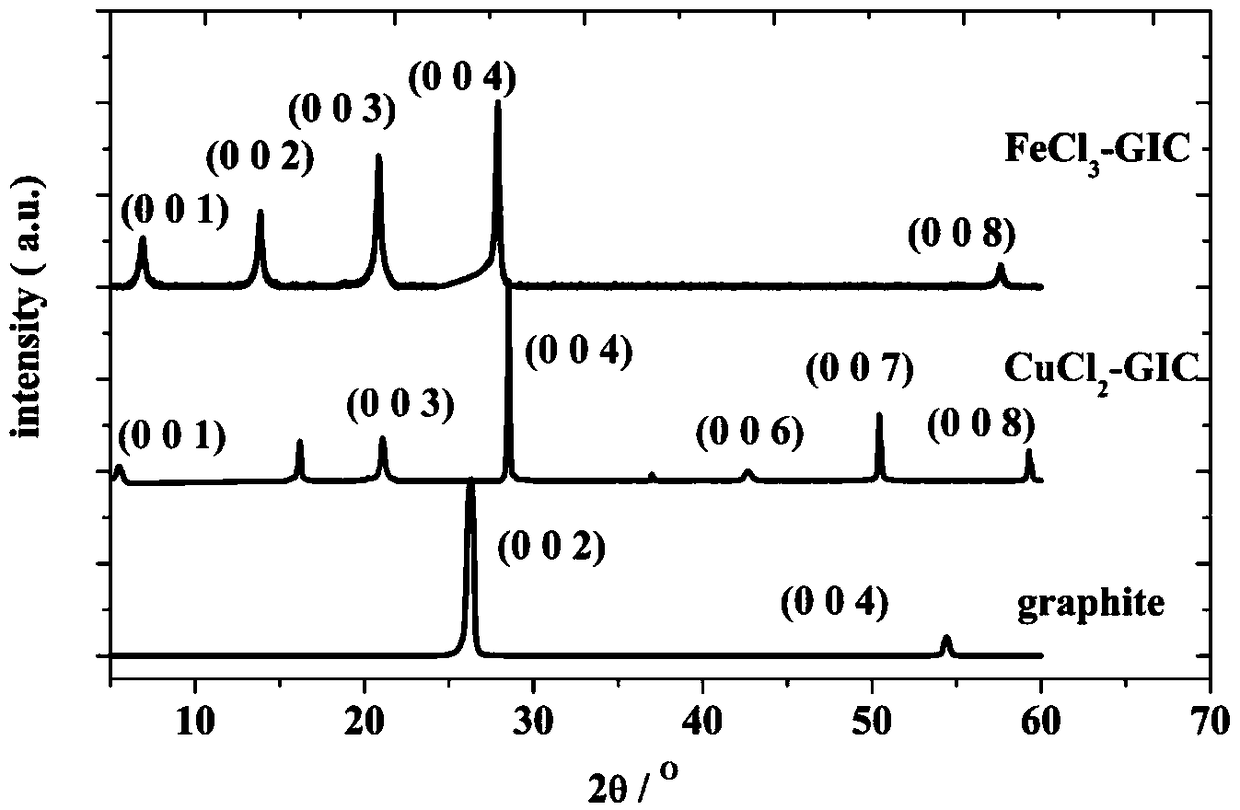
[0037] FeCl 3 -The preparation method of the GIC cathode material is: weigh 0.164g of natural flake graphite and 0.406g of FeCl in the glove box 3 , in which natural flake graphite and FeCl 3 The molar ratio is 5.44:1, and then put into a quartz test tube for fusion sealing. Put the sealed quartz test tube into a muffle furnace and heat it at 450°C for 1 hour, wash the product with deionized water until it has no color, and dry it in a drying oven at 70°C to obtain the product. Characteristic XRD diffraction peaks figure 2 Prove that FeCl 3 -GIC cathode material was successfully prepared. The electrochemical performance test was carried out after the aluminum-chlori...
specific Embodiment 3
[0039] With metal aluminum as the negative electrode, aluminum trichloride AlCl 3 and [EMIm]Cl as the electrolyte, with CoCl 2 -GIC is the positive electrode material, assembled into an aluminum-chloride mixed ion battery. The molar ratio of the electrolyte [EMIm]Cl:AlCl 3 =1:1. The preparation method is as in Example 1.
[0040] CoCl 2 -The preparation method of GIC cathode material is: CoCl 2 -GIC is made of natural flake graphite and anhydrous CoCl 2 Synthesized by molten salt method, CoCl 2 The molar ratio with graphite is 1:4n (n is the order), the reaction temperature is 660°C, and the reaction time is 12h. The experimental process is: first put the reactants into a heat-resistant quartz glass tube, mix them thoroughly and then vacuumize them for 5min. After the reaction is completed, the reaction tube is cooled with liquid nitrogen to liquefy the gas in it, and after taking it out, it is washed and dried to obtain the product. The electrochemical performance tes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com