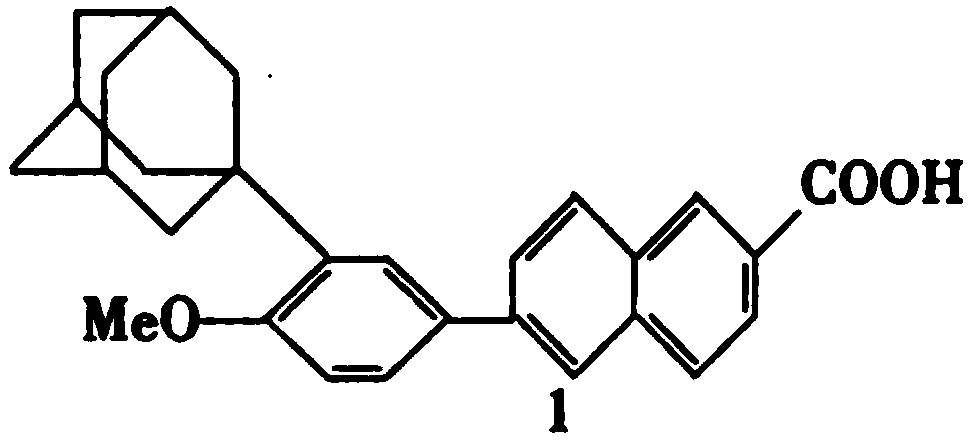
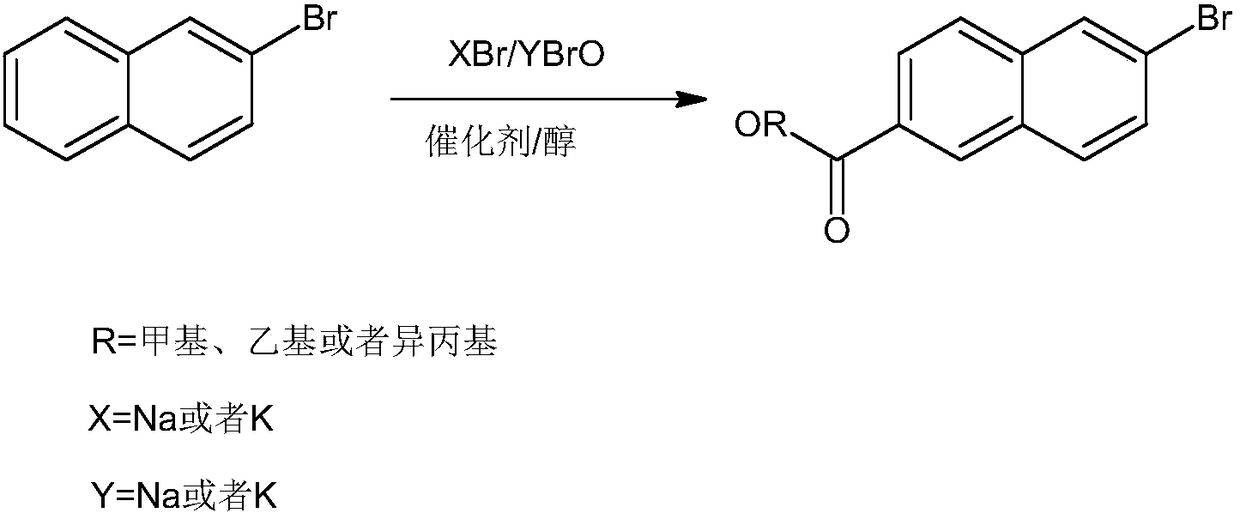
Method for synthesizing adapalene intermediate 6-bromo-2-naphthoate
A technology of adapalene and naphthoate, which is applied in the field of synthesis of adapalene intermediate 6-bromo-2-naphthoate, can solve the problems of low process yield and pollution discharge, and achieve reaction selection High solubility, less side effects, good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The synthetic method of 6-bromo-2-naphthoic acid methyl ester, comprises the following steps:
[0031] 1) Mix 2-naphthoic acid and methanol, add the catalyst, stir and pass in argon, control the pressure to 6 atmospheres, control the temperature to 150°C, and keep it for 60 minutes; start adding the mixture of sodium bromide and benzenesulfonic acid dropwise Aqueous solution, when the dripping volume reaches 8% of the solution volume, start to drop the sodium hypobromite aqueous solution; control the same dropping speed of the two solutions, and the total dropping time is 110min; after the dropping, the system is heated to 170°C , pressurized to 15 atmospheres to continue the reaction for 6h to end.
[0032] The catalyst is obtained by the following method: uniformly grinding nano-titanium dioxide and nano-silicon dioxide, soaking in hydrochloric acid with a mass concentration of 8% for 5 hours, filtering, washing with water, drying in the air, and drying at 300°C.
[...
Embodiment 2
[0036] The synthetic method of 6-bromo-2-naphthoic acid isopropyl ester comprises the following steps:
[0037] 1) Mix 2-naphthoic acid and isopropanol, add the catalyst, stir and pass nitrogen, control the pressure to 4 atmospheres, control the temperature to 130 ° C, and keep it for 50 minutes; start dropping potassium bromide and benzenesulfonic acid Mix the aqueous solution, and when the dropwise addition volume reaches 5% of the solution volume, start to drop the aqueous solution of potassium hypobromite; control the same dropping speed of the two solutions, and the total dropping time is 90min; The pressure was raised to 11 atmospheres and the reaction was continued for 5h to end.
[0038] The catalyst is obtained by the following method: uniformly grinding nano-titanium dioxide and nano-silicon dioxide, soaking in hydrochloric acid with a mass concentration of 5% for 3 hours, filtering, washing with water, drying in the air, and drying at 250°C.
[0039] The molar rati...
Embodiment 3
[0042] The synthetic method of 6-bromo-2-naphthoic acid ethyl ester, comprises the following steps:
[0043] 1) Mix 2-naphthoic acid and ethanol, add the catalyst, stir and pass in argon, control the pressure to 7 atmospheres, control the temperature to 155°C, and keep it for 70 minutes; start to add the mixture of sodium bromide and benzenesulfonic acid dropwise Aqueous solution, when the dropping volume reaches 10% of the volume of the solution, start to drop the sodium hypobromite aqueous solution; control the dropping speed of the two solutions to be the same, and the total dropping time is 150min; after the dropping, the system is heated to 180°C , the pressure was increased to 17 atmospheres and the reaction was continued for 7h to end.
[0044] The catalyst is obtained by the following method: uniformly grinding nano-titanium dioxide and nano-silicon dioxide, soaking in hydrochloric acid with a mass concentration of 10% for 6 hours, filtering, washing with water, drying...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com