Alveolar bone repair material containing BMP-2 as well as preparation method and application of alveolar bone repair material
A technology of BMP-2 and repair materials, applied in the field of regenerative medicine, can solve problems such as fast degradation, poor mechanical properties, and difficulty in achieving balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0036] The preparation of embodiment 1BMP-2
[0037] In order to facilitate the experiment process and quality control, the BMP-2 used in the experiment was prepared by the applicant:
[0038] According to the BMP-2 amino acid and gene sequence in Genbank, the BMP-2 gene sequence suitable for expression in Escherichia coli is constructed, and its nucleotide sequence (containing partial restriction site) is SEQ ID NO.1 in the sequence table; Insert the gene fragment into the pET plasmid vector after digestion, and transform the E. coli host after ligation; screen the positive clones and induce the expression of BMP-2; ferment, disrupt the bacteria, collect the inclusion bodies, lyse the inclusion bodies and refold the protein; anion and cation exchange layer BMP-2 was obtained by analysis and purification, and electrophoresis showed a single band of about 12kDa (consistent with the reported BMP-2), and the amino acid sequence of the product was SEQ ID NO.2 in the sequence listi...
Embodiment 2
[0040] The preparation of embodiment 2 modified chitosan
[0041] Chitosan modified with allyltrimethylammonium chloride:
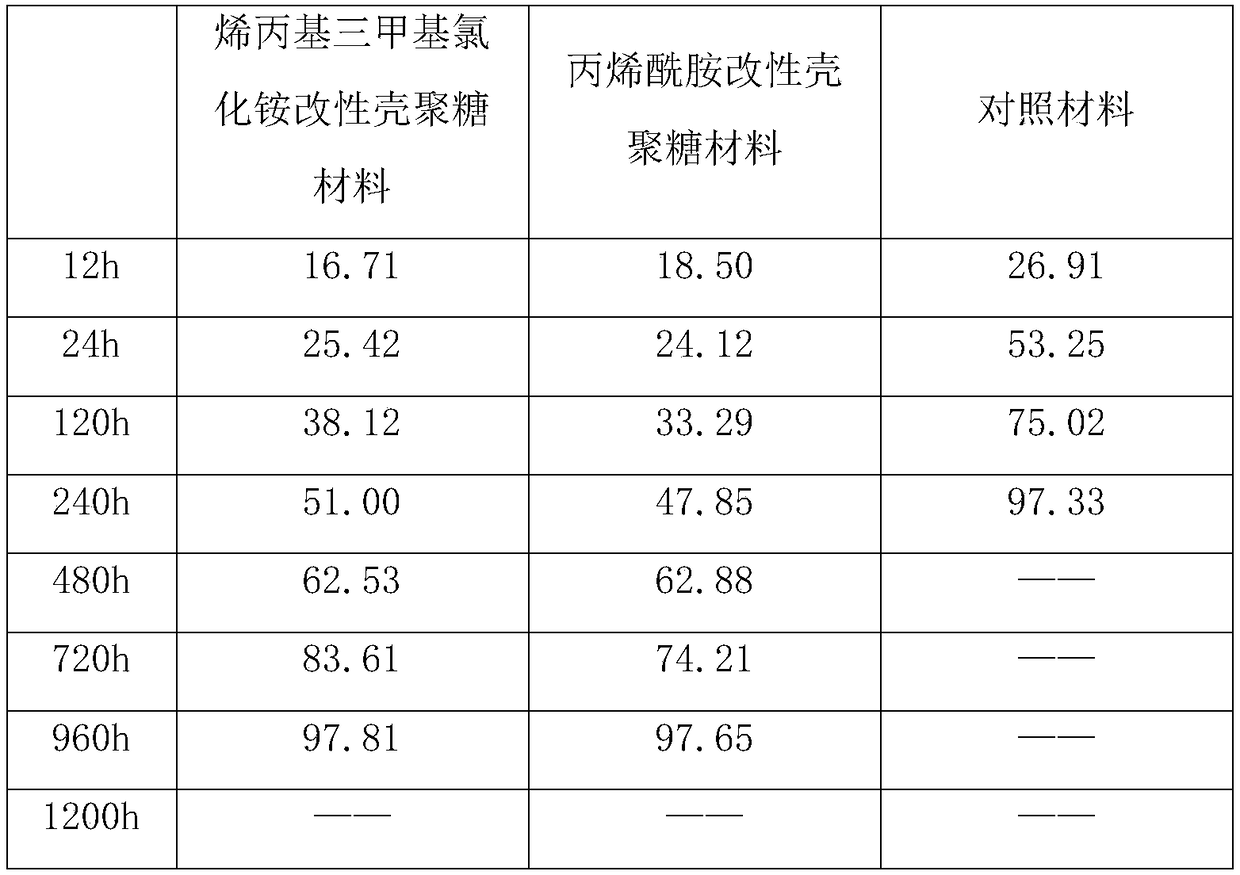
[0042] Dissolve 3g chitosan in 250ml 5% (volume) acetic acid solution; under argon protection, heat to 90°C, add 6ml 0.08mol / L cerium nitrate, react for 30min; add 9ml 50% (mass) allyltrimethyl Ammonium chloride aqueous solution, reacted for 2h; cooling, ethanol precipitation, washing, suction filtration, and vacuum drying to obtain allyltrimethylammonium chloride modified chitosan.
[0043] Product infrared spectrum detection shows 1415cm -1 (-NH), 1555cm -1 The characteristic absorption peak of (C=O) shows that allyltrimethylammonium chloride has been grafted on chitosan.
[0044]Chitosan modified with acrylamide:
[0045] Dissolve 3g chitosan solution in 250ml 5% (volume) acetic acid solution; under argon protection, heat to 50°C, add 5ml0.06mol / L cerium nitrate, react for 30min; add 8g acrylamide, react for 2h; cool, add Adjust the pH to 10 with ...
Embodiment 3
[0047] Preparation of Example 3 Alveolar Bone Restoration Material and Its Basic Properties
[0048] Alveolar bone repair materials of hydroxyapatite, allyltrimethylammonium chloride or acrylamide modified chitosan, hydroxypropyl methylcellulose, and BMP-2 polypeptide components (mass ratio 5000:2000 : 1500: 300) Store refrigerated at 4°C when not in use. Before use, mix the BMP-2 polypeptide with the calcium matrix evenly, dissolve the modified chitosan and hydroxypropyl methylcellulose in PBS buffer at pH 5 at a mass / volume concentration of 40%, and dissolve the BMP-2 polypeptide The mixture with the calcium base is added to the modified chitosan and hydroxypropyl methylcellulose solution, and mixed evenly to obtain the alveolar bone repair material. After the material is prepared, it needs to be molded and implanted within about 15 minutes.
[0049] The compressive strength of the newly prepared finished material is about 177MPa, the bending strength is about 250MPa, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| bending strength | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com