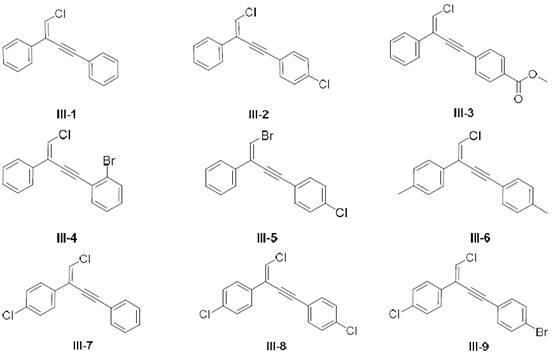
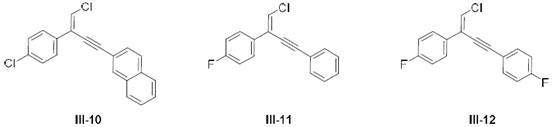
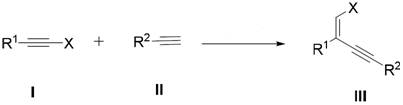
A kind of terminal alkene halogenated 1,3-enyne compound and its preparation and application
A compound, the technology of alkenyl halogenation, applied in the field of terminal alkenyl halogenation, can solve the problems of complex synthesis routes and few alternative methods, and achieve broad application prospects and good inhibitory selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of alkyne halide (I)
[0031] (1) Preparation of phenyl alkyne chloride (I-1)
[0032] The reaction formula is as follows:
[0033]
[0034] Add phenylacetylene (II-1) (2.042g, 20mmol), cesium carbonate (3.84g, 22mmol), tetra-n-butylammonium chloride (278mg, 1mmol) into a round-bottomed flask of carbon tetrachloride, at 70 The reaction was stirred and stirred at °C for 7 hours. After the reaction, add n-hexane (100 mL) to dilute, filter, collect the filtrate, use a rotary evaporator to evaporate the solvent under reduced pressure in a water bath at 40°C. Finally, column chromatography (mobile phase is petroleum ether) yielded a colorless target compound (I-1) with a yield of 83.0%. Its structure is characterized as follows:
[0035] 1 H NMR (600MHz, CDCl 3 )δ7.36-7.33(m,2H),7.24-7.20(m,3H). 13 C NMR (150MHz, CDCl 3 ) δ131.9,128.6,128.3,122.1,69.4,68.0.GC-MS(EI):m / z 136[M + ],138[(M+2) + ].
[0036] The preparation methods of ...
Embodiment 2
[0049] Example 2: Preparation of 1,3-enyne compound (III-1) with terminal alkenyl chloride
[0050] The reaction formula is as follows:
[0051]
[0052] Add IPrAuCl (5mol%, 9.3mg, 0.015mmol) in reaction bottle, NaBArF 4 (10mol%, 26.6.3mg, 0.03mmol), first dissolved with 2.0mL dichloroethane, and added phenylalkyne chloride (I-1) (41.0mg, 0.3mmol) into the reaction flask at 15°C, and started stirring , to which phenylacetylene (II-1) (30.6 mg, 0.3 mmol) was added, stirred and reacted at 15° C. for 10 hours, and the reaction process was monitored with a TLC plate. After the reaction, filter, wash the filter cake with dichloromethane (0.6mL×2), combine the filtrates, evaporate the solvent, and finally use column chromatography (mobile phase is n-hexane, Rf=0.5-0.7) to purify the terminal alkenes Chlorinated 1,3-enynes (III-1), yield 91%.
[0053] Its structure is characterized as follows:
[0054] 1 H NMR (600MHz, CDCl 3 )δ7.66(d,J=1.2Hz,1H),7.65-7.62(m,3H),7.42(dd,J=9....
Embodiment 3
[0055] Example 3: Preparation of 1,3-enyne compound (III-2) with terminal alkenyl chloride
[0056] The reaction formula is as follows:
[0057]
[0058] The phenylacetylene (II-1) (30.6mg, 0.3mmol) in Example 2 was replaced by p-chlorophenylacetylene (II-2) (41.0mg, 0.3mmol), other operations were the same as in Example 2, and finally the terminal Alkenyl-chlorinated 1,3-enynes (III-2), yield 91%. Its structure is characterized as follows:
[0059] 1 H NMR (600MHz, CDCl 3 )δ7.55(d, J=7.0Hz, 2H), 7.46(d, J=8.5Hz, 2H), 7.36-7.28(m, 5H), 6.82(s, 1H). 13 C NMR (150MHz, CDCl 3 )δ135.65, 134.92, 132.98, 128.74, 128.71, 128.65, 126.85, 126.18, 124.16, 121.19, 97.34, 85.64. GC-MS (EI): m / z 272 [M + ].IRν max (cm -1 ):3414,1627,1486,1388,1093,828,696.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com