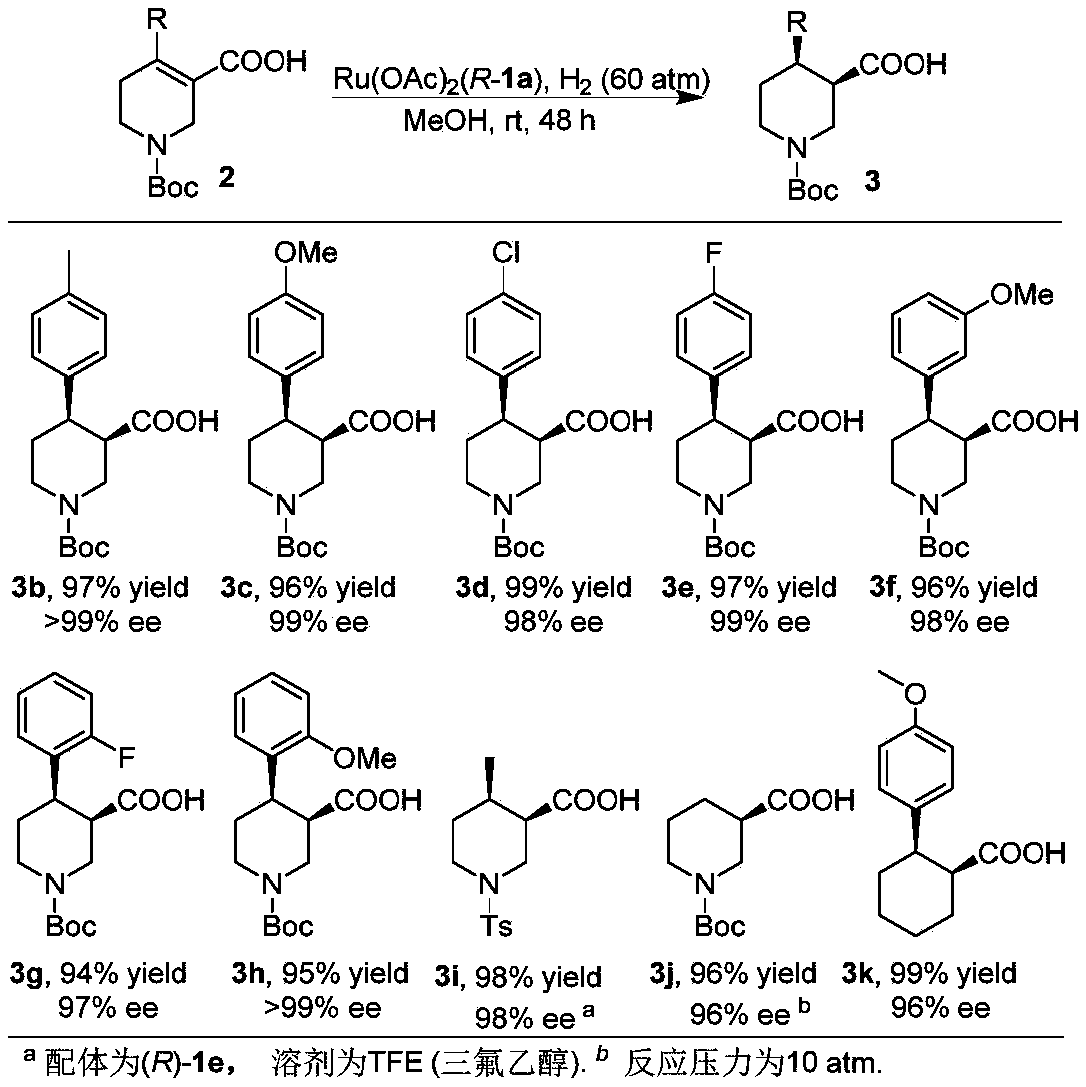
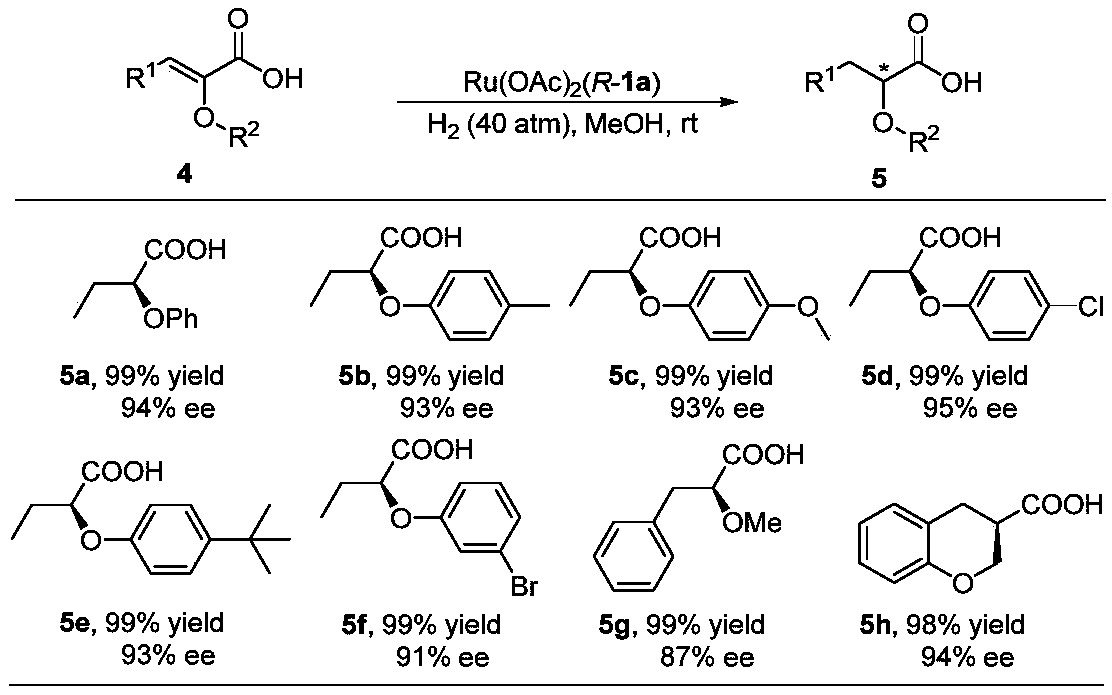
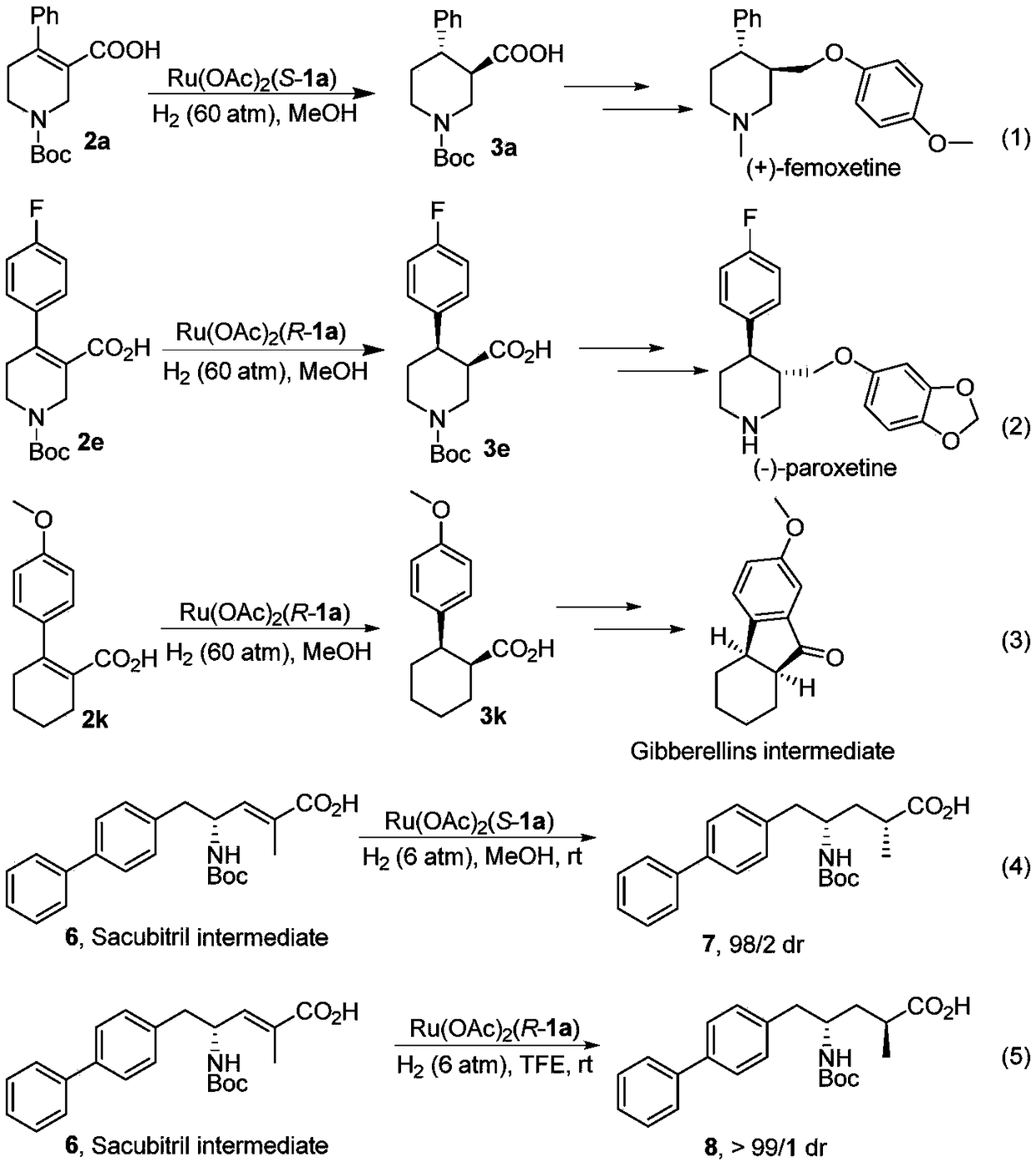
Oxa-spiral diphosphine ligand and application thereof in alpha, beta-unsaturated carboxylic acid asymmetric hydrogenation
A bisphosphine ligand and oxaspiro technology is applied in the direction of asymmetric synthesis, preparation of carboxylic acid by ozonation, and preparation of cyanide reaction. problems such as sexual bisphosphine ligands, to achieve the effect of high activity and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Catalyst Rh(1a)OAc 2 Preparation of:
[0052] in N 2 Under atmosphere, add [RuPhCl 2 ] 2 (25 mg, 0.05 mmol), ligand 1a (61 mg, 0.103 mmol), and then 2 mL of DMF was added. React at 100°C for 3h. After cooling to room temperature, 1.5 mL of anhydrous sodium acetate (0.111 g, 1.3 mmol) in methanol was added. After 20Min, deoxygenated deionized water was added. A gray solid precipitated from the reaction system, filtered, and the solvent and water were removed under reduced pressure to obtain the catalyst Rh(1a)OAc 2 (57 mg, yield = 71%).
Embodiment 2
[0054] Catalyst Rh(1a)(CF 3 CO) 2 Preparation of:
[0055] in N 2 Under atmosphere, add bis-(2-methallyl) cyclooct-1,5-diene ruthenium (32mg, 0.05mmol), ligand 1a (61mg, 0.103mmol) to a 10mL single-necked bottle, and then add 2 mL of acetone. React at 40°C for 0.5h. Then add trifluoroacetic acid (33 mg, 0.3 mmol), stir overnight at 40 ° C, remove the solvent under reduced pressure, then add 1 mL of petroleum ether, and filter to obtain the target product Rh (1a) (CF 3 CO) 2 (81 mg, yield = 88%).
Embodiment 3
[0057] Synthesis of (3R,4R)-1-(tert-butoxycarbonyl)-4-phenyl-3-carboxylic acid 3a:
[0058] in N 2 Under atmosphere, add 2a (0.1mmol), catalyst Ru(1a)OAc to the hydrogenation vial 2 (0.8mg, 0.001mmol) and 1mL of methanol. After 24 h under a hydrogen atmosphere of 60 atm, all the raw materials were converted into products. 29.0mg, product yield=95%,>99%ee, [α] 25 D =+38.0 (c=0.5, CHCl 3 ), yellow oil. 1 H NMR (400MHz, CDCl 3 )δ7.29-7.24(m,2H,Ar),7.23-7.17(m,3H,Ar),4.44(d,J=12.7Hz,1H,CH 2 ), 4.26 (d, J=9.0Hz, 1H, CH 2 ), 3.16(d, J=11.1Hz, 1H, CH), 3.01-2.82(m, 3H, CH 2 ),2.55(dt,J=12.0,8.6Hz,1H,CH),1.68(dd,J=13.0,2.8Hz,1H,CH 2 ),1.39(s,9H,CH 3 ). 13 C NMR (101MHz, CDCl 3 )δ176.9, 154.7, 142.1, 128.3, 127.4, 126.6, 79.8, 46.1, 45.2, 43.8, 43.0, 28.2, 25.6. HRMS (ESI) calcd.for C 17 h 22 NO 4 [M-H] - :304.1554, Found: 304.1556.HPLC conditions: Daicel AD-3, injection volume 2μL (c=1mg / mL), Hexane / IPA=97 / 3, 1.0mL / Min, 208nm, t R (major) = 29.6Min,t R (minor) = 31....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com