Method for synthesizing acrylic acid
An acrylic acid and complex technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylates, etc., can solve the problems of expensive catalysts, difficult to control catalyst loss, harsh reaction conditions, etc., and achieve catalytic activity and reaction. High selectivity, ease of scale-up and industrial application, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
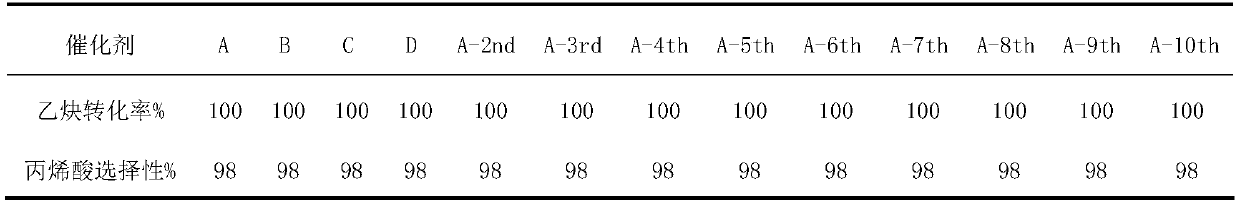
Image
Examples
Embodiment 1
[0023] Preparation of acid functionalized ionic liquid palladium phosphine ligand complex catalyst supported by NaY molecular sieve:
[0024] a. Add 10g NaY molecular sieve and 25ml methyl imidazole to a 250ml Erlenmeyer flask in turn, stir at room temperature for 8h, then add 75ml of decane bromide into the Erlenmeyer flask, stir at a temperature of 50°C for 12h, filter , washed 3 times with 30ml absolute ethanol, dried to obtain 8g white solid powder;
[0025] b. Add 8 g of white solid powder obtained in step a, 30 ml of organic solvent ethyl acetate into a 100 ml conical flask in turn, add 2 g of organic acid p-toluenesulfonic acid, stir for 12 hours at a temperature of 50 ° C, filter, and use 30 Washed 3 times with absolute ethanol, dried to obtain 9g white solid powder;
[0026] c. Add 9g of white solid powder obtained in step b and 30ml of distilled water to a 100ml Erlenmeyer flask in turn, and add 2ml of 0.28mol / L H 2 PdCl 4 Solution, after stirring continuously for...
Embodiment 2
[0030] Preparation of acid functionalized ionic liquid palladium phosphine ligand complex catalyst supported by NaY molecular sieve:
[0031] a. Add 15g NaY molecular sieve and 50ml methyl imidazole to a 250ml Erlenmeyer flask in turn, stir at room temperature for 12h, then add 120ml of decane bromide into the Erlenmeyer flask, stir at a temperature of 80°C for 6h, filter , washed 3 times with 60ml absolute ethanol, dried to obtain 11g white solid powder;
[0032] b. Add 11g of white solid powder obtained in step a, 30ml of organic solvent acetone to a 100ml Erlenmeyer flask successively, add 4g of organic acid trifluoromethanesulfonic acid, stir at a temperature of 80°C for 6 hours, filter, and use 60ml of Washed with water and ethanol for 3 times, dried to obtain 12g white solid powder;
[0033] c. Add 12g of white solid powder obtained in step b and 30ml of distilled water to a 100ml Erlenmeyer flask, and add 4.8ml of 0.28mol / L H 2 PdCl 4 After stirring continuously for ...
Embodiment 3
[0037] Preparation of acid functionalized ionic liquid palladium phosphine ligand complex catalyst supported by NaY molecular sieve:
[0038] a. Add 12g NaY molecular sieve and 35ml methyl imidazole to a 250ml conical flask in turn, stir at room temperature for 10 hours, then add 100ml bromodecane to the conical flask, and stir for another 8 hours at a temperature of 60°C , filtered, washed 3 times with 45ml of absolute ethanol, and dried to obtain 9.5g of white solid powder;
[0039] b. Add 9.5g of white solid powder obtained in step a and 30ml of organic solvent tetrahydrofuran into a 100ml conical flask in turn, add 3g of organic acid p-toluenesulfonic acid, stir for another 10 hours at a temperature of 70°C, filter, and use 40ml Wash with absolute ethanol for 3 times, and dry to obtain about 10.5g of white solid powder;
[0040] c. Add 10.5g of white solid powder obtained in step b and 30ml of distilled water to a 100ml Erlenmeyer flask in turn, and add 3.6ml of 0.28mol / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com