Hydrofluoroether compound and preparation method and application thereof
A compound, hydrofluoroether technology, applied in the field of hydrofluoroether
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
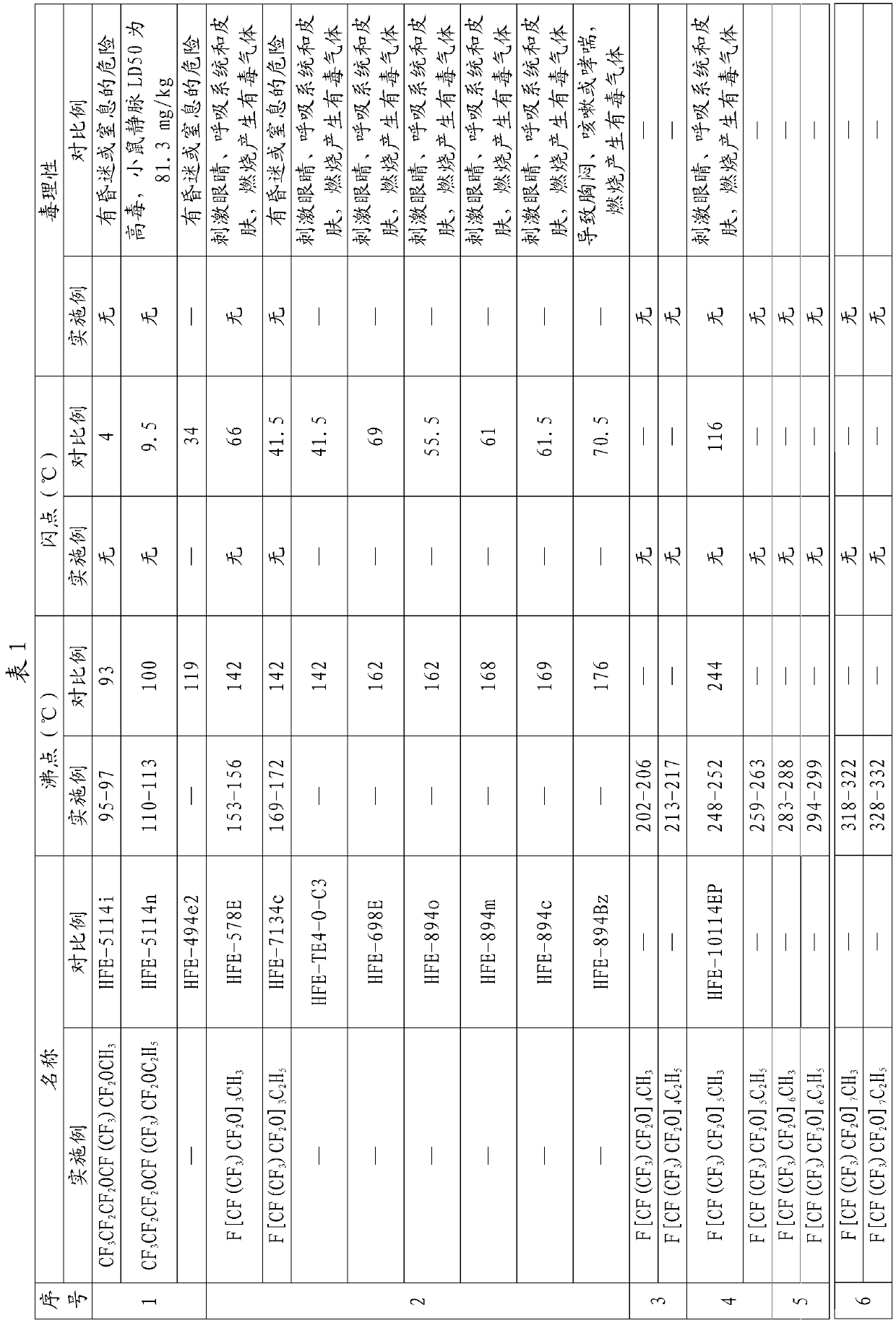
[0066] Example 1CF 3 CF 2 CF 2 OCF (CF 3 ) CF 2 OCH 3 preparation of
[0067] The present invention will be further illustrated below in conjunction with non-limiting examples and test methods. All parts, percentages and ratios are by weight unless otherwise indicated. Solvents and other reagents used were from Sigma-Aldrich Chemical Company unless otherwise stated.
[0068] 1.1: CF 3 CF 2 CF 2 OCF (CF 3 ) Preparation of C(O)F
[0069] CF 3 CF 2 CF 2 OCF (CF 3 )C(O)F, hydrofluoroether CF 3 CF 2 CF 2 OCF (CF 3 ) CF 2 OCH 3 The precursor of , is synthesized by the dimerization of hexafluoropropylene oxide. In the reactor, add hexadecyltrimethylammonium bromide (10.0 grams) and acetonitrile (2.5 liters), at 25 DEG C and under stirring condition, pass into hexafluoropropylene oxide (830 liters) in the reactor for 3 hours g, 5.0 mol). The product of the lower acyl fluoride phase is collected, mainly composed of hexafluoropropylene oxide dimer and hexafluorop...
Embodiment 2
[0072] Example 2CF 3 CF 2 CF 2 OCF (CF 3 ) CF 2 OC 2 h 5 preparation of
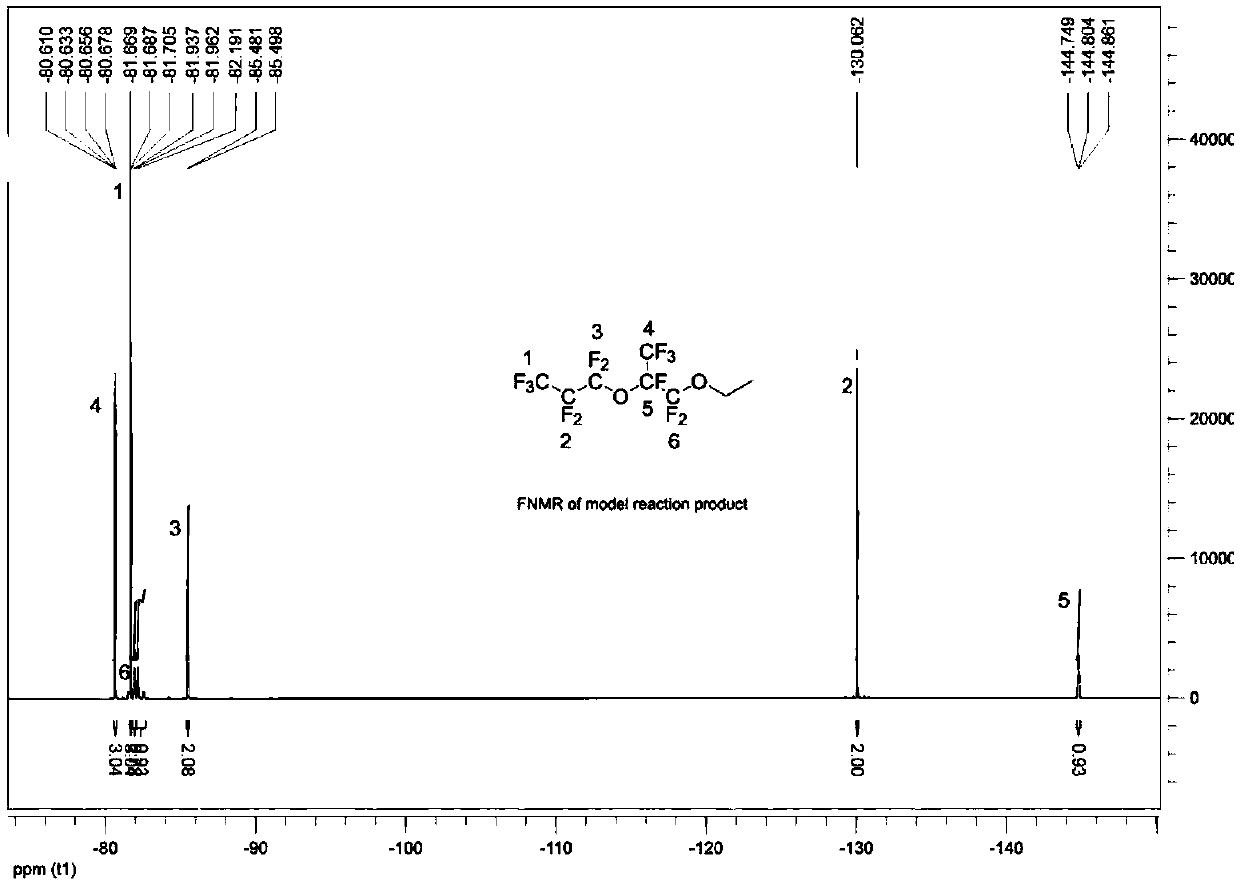
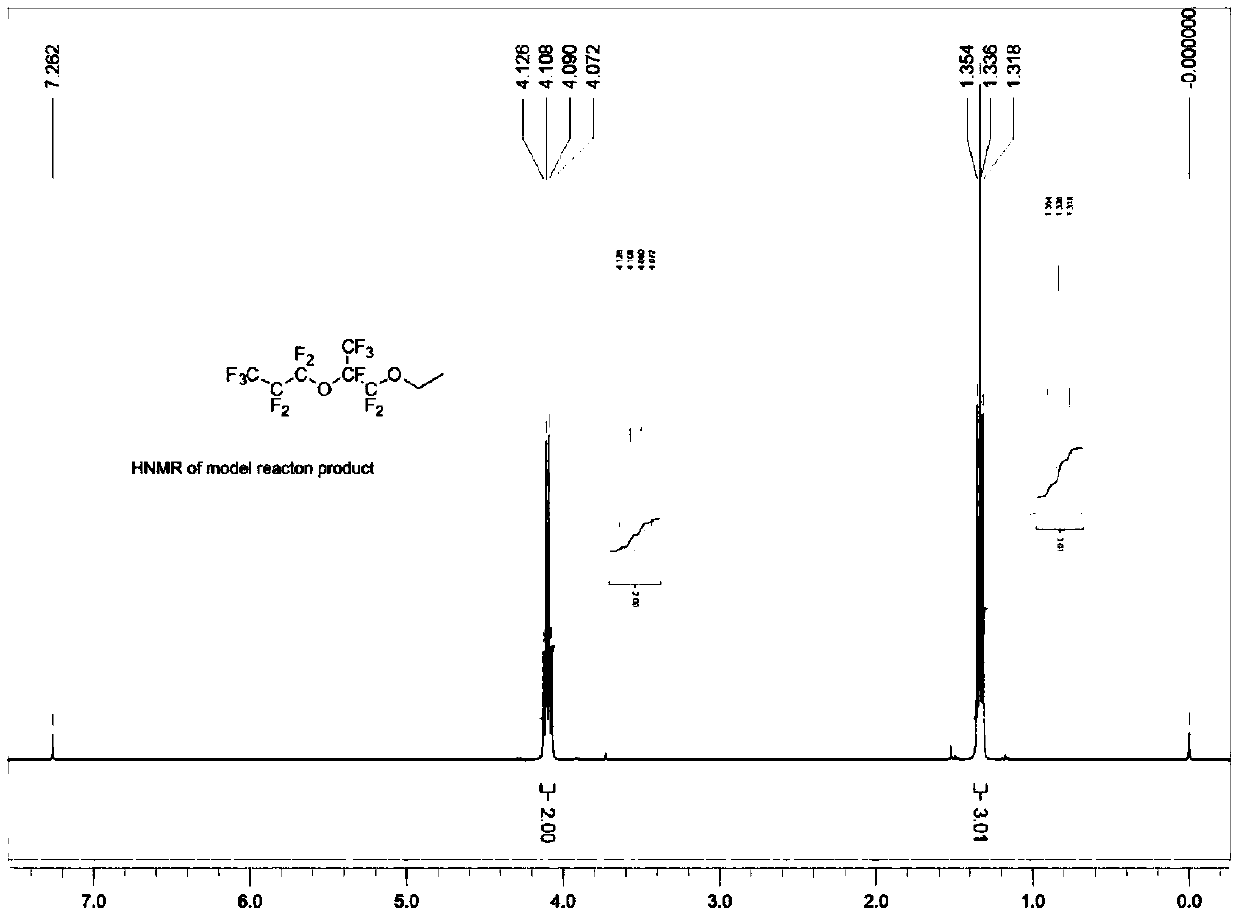
[0073] With reference to Example 1, in the reactor, add spray-dried potassium fluoride (37.2 grams, 0.64 moles, purchased from Aldrich Chemical Company, through further drying at 120 ° C), anhydrous diglyme ( 320 grams), hexafluoropropylene oxide dimer (157.2 grams, 0.48 mole, the gas chromatography records the purity as 99%), triethylamine (4.82 grams, 0.048 mole), trioctylmethyl ammonium chloride ( 2.00 g, 0.005 mol) and diethyl sulfate (86.2 g, 0.56 mol), the mixture was heated to 54°C and maintained for 14 hours. After cooling down to room temperature, enough isopropanol was added to convert all unreacted hexafluoropropylene oxide dimer into ester, and stirred for 30 minutes. Water was added, the lower product was separated and collected, and washed once with cold water to obtain 188.0 g of crude product. Among them, CF 3 CF 2 CF 2 OCF (CF 3 ) CF 2 OC 2 h 5 、CF 3 CF 2 CF 2 OCF (CF ...
Embodiment 3F
[0074] Embodiment 3F [CF (CF 3 ) CF 2 O] 3 CH 3 preparation of
[0075] 3.1:F[CF(CF 3 ) CF 2 O] 2 CF(CF 3 ) Preparation of C(O)F
[0076] F[CF(CF 3 ) CF 2 O] 2 CF(CF 3 )C(O)F, hydrofluoroether F[CF(CF 3 ) CF 2 O] 3 CH 3 The precursor of , is synthesized by the trimerization of hexafluoropropylene oxide. In the reactor, add cetyltrimethylammonium bromide (25.0 grams) and acetonitrile (2.5 liters), at 30 ℃ and under stirring condition, pass into hexafluoropropylene oxide ( 830 g, 5.0 mol). The acyl fluoride phase product in the lower layer is collected, mainly composed of hexafluoropropylene oxide dimer and hexafluoropropylene oxide trimer, and the yield of hexafluoropropylene oxide trimer is 80%. The crude product was further purified by distillation to obtain a hexafluoropropylene oxide trimer product with a purity of 99% as determined by gas chromatography, which was used in the next step of the alkylation reaction. with infrared spectroscopy and 19 F NMR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com