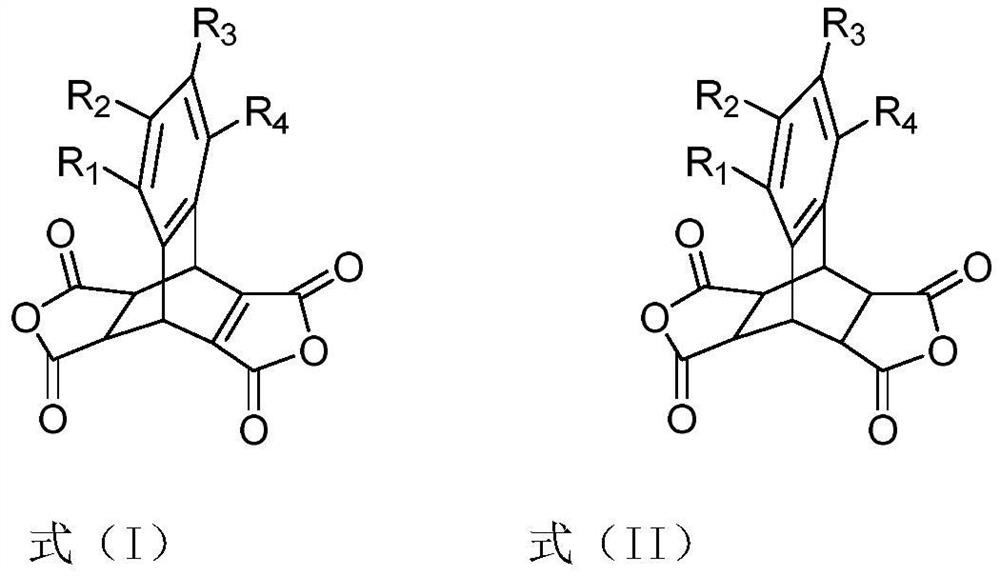
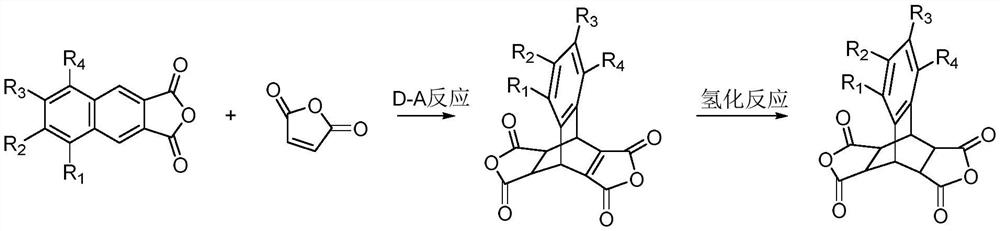
A dianhydride containing butterfly structure and its synthesis method and polyimide synthesized based on the dianhydride
A technology of polyimide and dianhydride, applied in the field of polyimide, can solve the problems of harsh storage conditions, inconvenient transportation and storage, and fragile film processing of polymers, achieve excellent mechanical properties, and increase glass transition temperature , Improve the effect of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
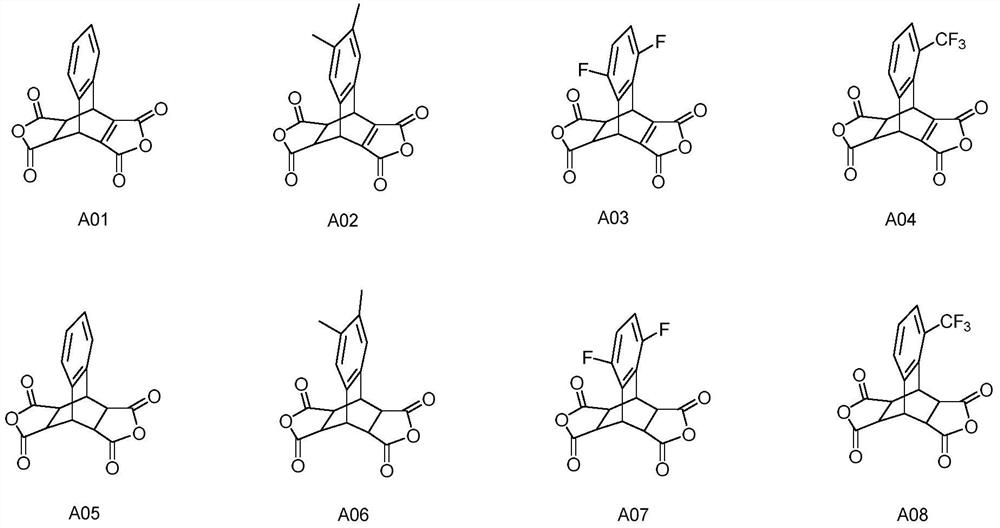
[0036] The preparation of embodiment 1 compound A01
[0037]
[0038] The preparation of compound A01: In a 1000mL three-necked flask, under nitrogen protection, add 2,3-naphthalene dioic anhydride (9.9g, 0.05mol), butenedioic anhydride (147.1g, 1.5mol), 300mL dry chlorobenzene, control The internal temperature is 100-105°C, and the reaction is kept for 72 hours. Slowly lower the temperature to below 60°C, change the reaction system to a vacuum distillation device, 500-600Pa, bath temperature 55-60°C, remove the solvent under reduced pressure until there is no fraction, further increase the bath temperature to 110-120°C, and further decompress and remove the solvent Butenedioic anhydride was reduced to no fraction, and 15.1 g of a light brown viscous solid was obtained. Add 60g of acetic anhydride to the above reaction crude product, heat up to 60-65°C, stir and beat for 5.0hrs, then slowly cool down to 0-5°C, use nitrogen for pressure filtration, transfer the filter cake ...
Embodiment 2
[0041] The preparation of embodiment 2 compound A05
[0042]
[0043] Preparation of compound A05: In a 500mL autoclave, add the above-mentioned compound A01 (5.9g, 0.02mol), Pd / C (1g, water content 56%, produced by Shaanxi Ruike New Materials) and 100g THF, fully replace with nitrogen, control The pressure is 30-40atm, the internal temperature is 55-60°C, after 8.0hrs of heat preservation reaction, it is lowered to 20-25°C, and the catalyst Pd / C is filtered out by suction filtration, and the solvent is removed under reduced pressure to obtain an off-white solid. Add 24g of acetic anhydride to the above reaction crude product, heat up to 60-65°C, stir and beat for 5.0hrs, then slowly cool down to 0-5°C, use nitrogen for pressure filtration, transfer the filter cake directly to a 100mL single-necked flask, vacuum 100 ~200Pa, bath temperature 115~120°C, and drying under reduced pressure for 12.0 hours to obtain 4.3g of off-white solid powder, that is, compound A05, with a yie...
Embodiment 3
[0046] The preparation of embodiment 3 compound A02
[0047]
[0048]Preparation of Compound A02: In a 1000mL three-neck flask, under nitrogen protection, add 6,7-dimethyl-2,3-naphthalene dicarboxylic anhydride (11.3g, 0.05mol), butenedioic anhydride (98.0g, 1.0mol) , 300mL of dry cyclohexane, control the internal temperature at 65-70°C, and keep it warm for 60 hours. Slowly lower the temperature to below 35°C, change the reaction system to a vacuum distillation device, 500-600Pa, bath temperature 30-35°C, remove the solvent under reduced pressure until there is no distillate, further increase the bath temperature to 110-120°C, and further decompress Butenedioic anhydride was reduced to no fraction, and 16.6 g of a light brown viscous solid was obtained. Add 48g of acetic anhydride to the above reaction crude product, heat up to 60-65°C, stir and beat for 4.0hrs, then slowly cool down to 0-5°C, use nitrogen for pressure filtration, transfer the filter cake directly to a 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com