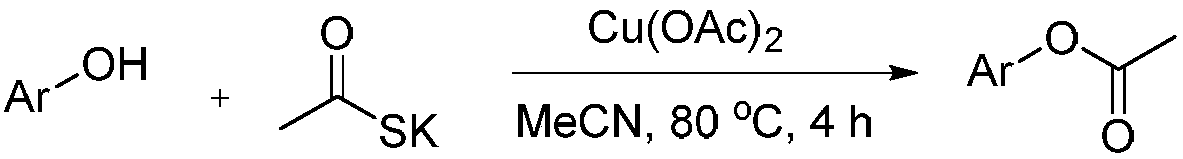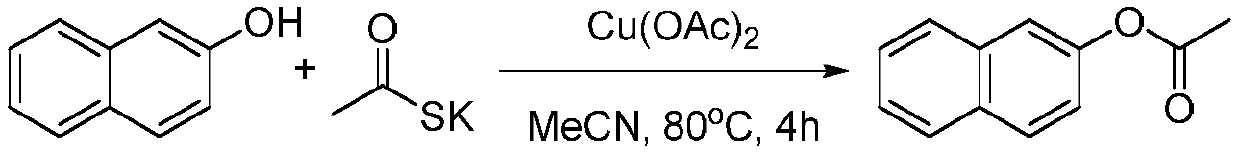Method for synthesizing phenyl acetate derivatives
A technology of phenyl acetates and synthetic methods, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of expensive catalysts, restricting industrial development, and difficult storage, etc., and achieve substrate expansion Wide-ranging, lucrative, and easy-to-manage effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the synthesis of acetic acid-2-naphthyl ester
[0016]
[0017] Add β-naphthol (43.2 mg), potassium thioacetate (102.6 mg), Cu(OAc) to a 25 mL reaction tube 2 (12.0 mg), acetonitrile (2 mL), and magnetically stirred at 80° C. for 4 hours. After the reaction is finished, extract with ethyl acetate, collect the organic phase, evaporate most of the solvent under reduced pressure, and use petroleum ether: ethyl acetate (10:1) as eluent to carry out column chromatography for the remaining mixed solution. After purification, the target product was obtained as white needle crystals, 51 mg, yield 92%.
[0018] Its NMR data are as follows:
[0019] 1 H NMR (300MHz, CDCl 3 )δ=7.91~7.87(m,3H),7.61(s,1H),7.53-7.51(t,2H),7.30-7.27(t,3H),2.40(s,3H);
[0020] 13 C NMR (75MHz, CDCl 3 ) δ = 169.62, 146.70, 134.75, 128.15, 126.87, 126.39, 126.13, 125.50, 125.14, 121.22, 118.18, 21.09.
Embodiment 2
[0021] Embodiment 2: the synthesis of acetic acid-1-naphthyl ester
[0022]
[0023] Add α-naphthol (43.2 mg), potassium thioacetate (102.6 mg), Cu(OAc) to a 25 mL reaction tube 2 (12.0 mg), acetonitrile (2 mL), and magnetically stirred at 80° C. for 4 hours. After the reaction is finished, extract with ethyl acetate, collect the organic phase, evaporate most of the solvent under reduced pressure, and use petroleum ether: ethyl acetate (10:1) as eluent to carry out column chromatography for the remaining mixed solution. After purification, the target product was obtained as colorless needle crystals, 46 mg, and the yield was 83%.
[0024] Its NMR data are as follows:
[0025] 1 H NMR (300MHz, CDCl 3 )δ=7.93~7.91(m,2H), 7.81~7.78(m,1H), 7.58~7.49(m,3H), 7.31~7.29(d,1H), 2.51(s,3H);
[0026] 13 C NMR (75MHz, CDCl 3 ) δ = 169.78, 148.42, 133.85, 131.56, 129.53, 127.88, 127.75, 126.67, 125.82, 121.25, 118.65, 21.30.
Embodiment 3
[0027] Embodiment 3: the synthesis of acetic acid-2-methylphenyl ester
[0028]
[0029] Add o-cresol (32.4 mg), potassium thioacetate (102.6 mg), Cu(OAc) to a 25 mL reaction tube 2 (12.0 mg), acetonitrile (2 mL), and magnetically stirred at 80° C. for 4 hours. After the reaction is finished, extract with ethyl acetate, collect the organic phase, evaporate most of the solvent under reduced pressure, and use petroleum ether: ethyl acetate (10:1) as eluent to carry out column chromatography for the remaining mixed solution. After purification, the target product was obtained as a white solid, 27 mg, and the yield was 60%.
[0030] Its NMR data are as follows:
[0031] 1 H NMR (300MHz, CDCl 3 )δ=7.27~7.17(m,3H),7.03(d,J=7.6Hz,1H),2.35(d,J=2.5Hz,3H),2.2(s,3H);
[0032] 13 C NMR (75MHz, CDCl 3 ) δ = 169.37, 149.40, 131.20, 130.16, 127.00, 126.13, 121.92, 20.89, 16.20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com