Catalyst for producing formic acid by electroreduction of carbon dioxide and preparation method thereof
A technology of carbon dioxide and electrocatalyst, which is applied in the field of electrocatalysis, can solve the problems of difficult breakthrough of formic acid generation rate, reduction of formic acid Faradaic efficiency, sensitivity of current and potential, etc., and achieves the advantages of simple preparation method, high selectivity and high reactivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
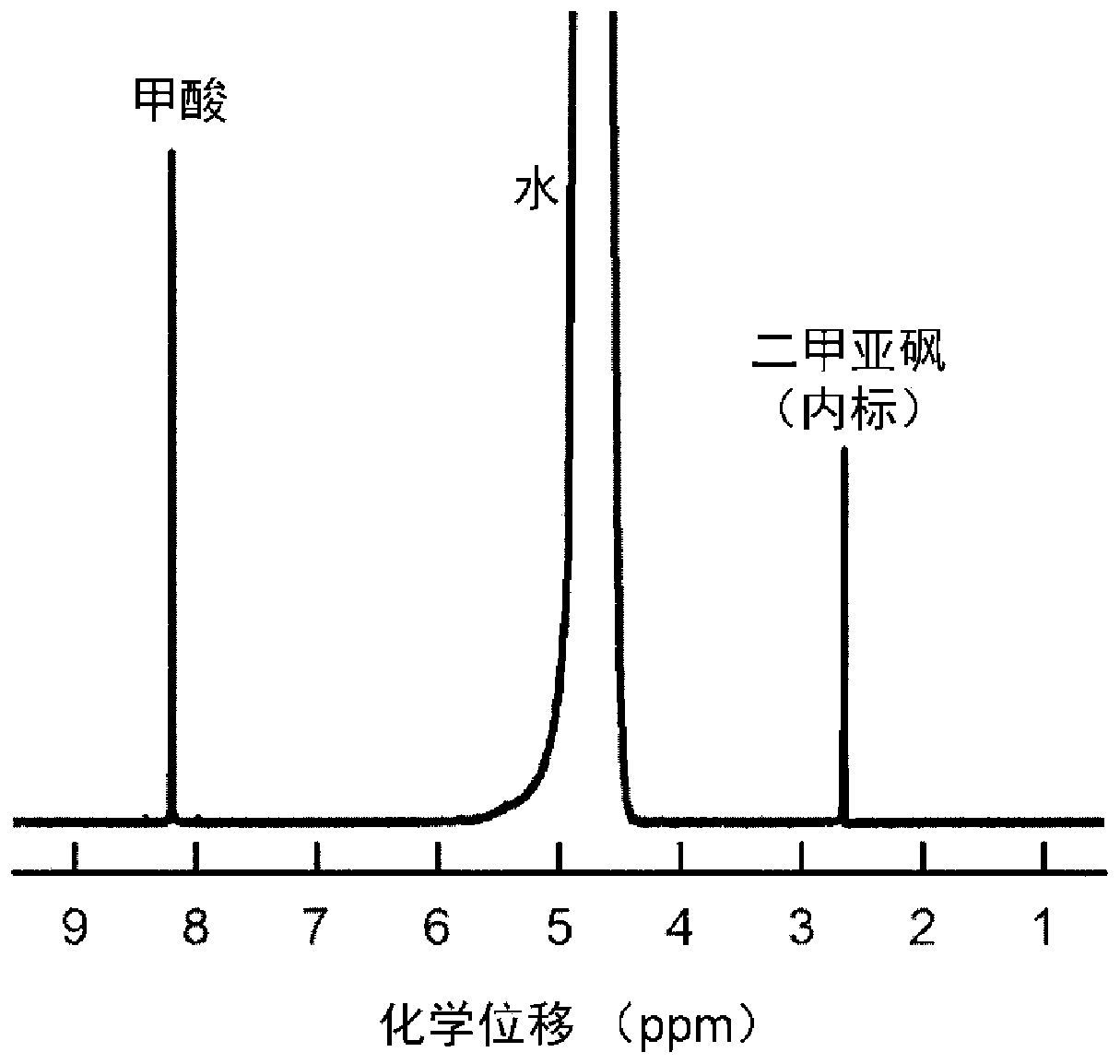
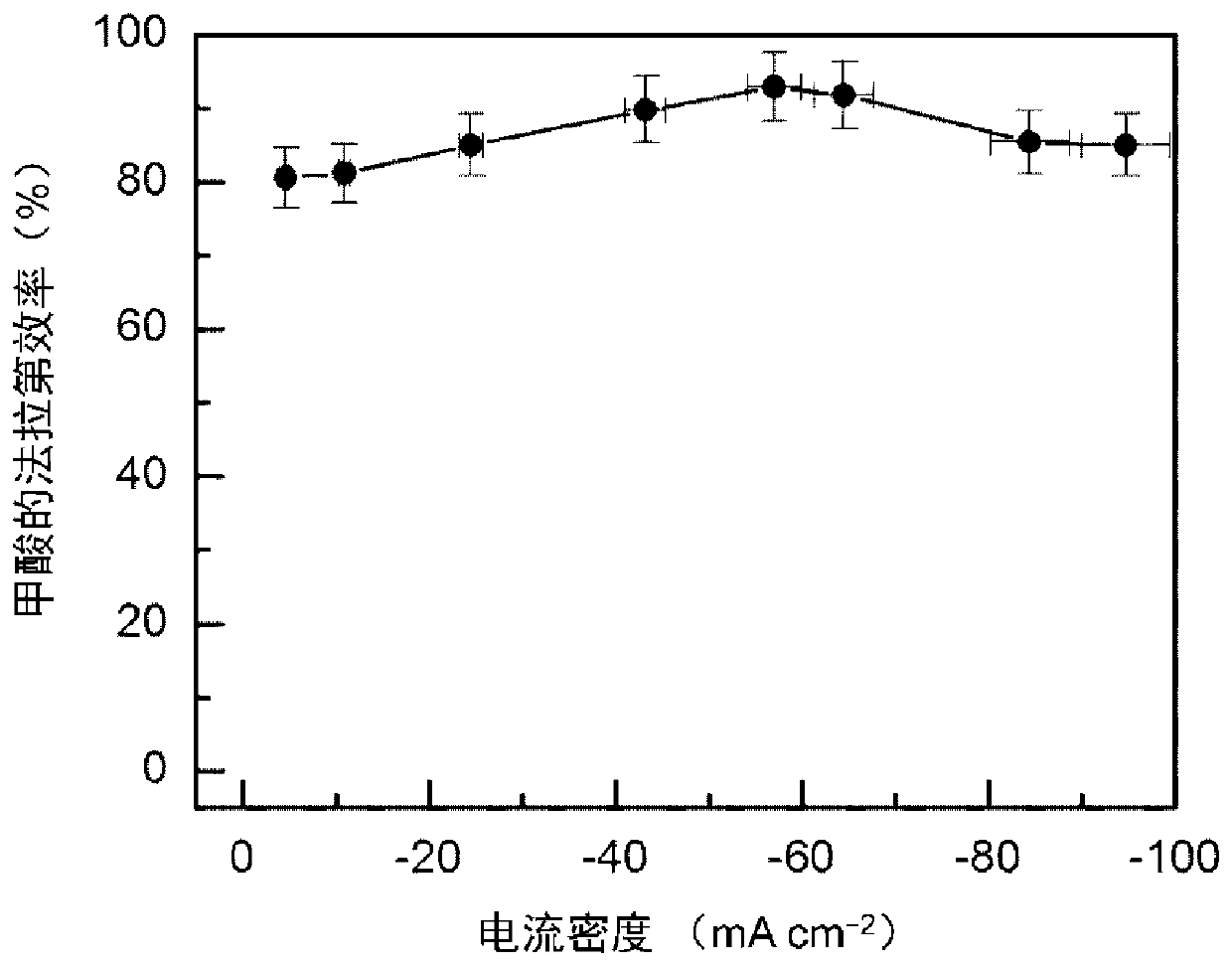
[0032] 16μmol thioacetamide and 0.4mmol InCl 3 Dissolve in 15mL DMF, stir vigorously for 15min, then transfer to a 25mL Teflon-lined stainless steel autoclave, and place in a clean 1×3cm 2 Carbon paper, sealed, heat-treated at 150°C for 12h; after cooling, the carbon paper was taken out, washed with deionized water, and dried to obtain S-doped In 2 o 3 Precursor; final in 0.5M KHCO 3 Electroreduction at -0.98V vs.RHE in the electrolyte for 5min yields a carbon paper-supported 4.9mol% S-doped In metal electrocatalyst (S-In), such as figure 1 Shown is a scanning electron microscope image of a 4.9% sulfur-doped metal indium electrocatalyst. The particle size of the sulfur-doped metal indium is about 130nm, and it is evenly loaded on the carbon fiber of the carbon paper. The catalyst is used as the cathode, the Pt sheet is used as the anode, and the saturated calomel electrode is used as the reference electrode, and the reaction is carried out in an H-type electrolytic cell. Bo...
Embodiment 2
[0035] 16μmol thioacetamide and 0.4mmol InCl 3 Dissolve in 15mL DMF, stir vigorously for 15min; then transfer to a 25mL PTFE-lined stainless steel autoclave, put in a clean 1×3cm 2 Carbon paper, sealed, heat-treated at 150°C for 12h; after cooling, the carbon paper was taken out, washed with deionized water, and dried to obtain S-doped In 2 o 3 Precursor; final in 0.5M KHCO 3 The carbon paper-supported 4.9mol% S-doped In metal electrocatalyst (S-In) was obtained by electroreduction at -0.98V vs. RHE in the electrolyte for 5min. The catalyst is used as the cathode, the Pt sheet is used as the anode, and the saturated calomel electrode is used as the reference electrode; the reaction is carried out in an H-type electrolytic cell, and both the cathode chamber and the anode chamber are 30mL 0.5M CsHCO 3 Electrolyte; carbon dioxide at a certain 20mLmin -1 The flow rate is passed into the catholyte, and the potential reaction of -0.98V vs. RHE is applied for 1h; the current dens...
Embodiment 3
[0037] 16μmol thioacetamide and 0.4mmol InCl 3Dissolve in 15mL DMF, stir vigorously for 15min; then transfer to a 25mL PTFE-lined stainless steel autoclave, put in a clean 1×3cm 2 Carbon cloth, sealed, heat treatment at 150°C for 12h; after cooling, the carbon cloth was taken out, washed with deionized water, and dried to obtain S-doped In 2 o 3 Precursor; final in 0.5M KHCO 3 The carbon cloth-supported 4.9mol% S-doped In metal electrocatalyst (S-In) was obtained by electroreduction at -0.98V vs. RHE in the electrolyte for 5 minutes. The catalyst is used as the cathode, the Pt sheet is used as the anode, and the saturated calomel electrode is used as the reference electrode; the reaction is carried out in an H-type electrolytic cell, and both the cathode chamber and the anode chamber are 30mL 0.5M KHCO 3 Electrolyte; carbon dioxide at a certain 20mL min -1 The flow rate is passed into the catholyte, and the potential reaction of -0.98V vs. RHE is applied for 1h; the curren...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com