Method for preparing fluacrypyrim
A technology for pyrimidine and trifluoromethylpyrimidine, which is applied in the field of synthesis of the acaricide pyrimidine, can solve the problems of difficult recovery and reuse of organic solvents, large usage of organic solvents, limited capacity of chromatographic columns, etc. Low, shorten the reaction time, the effect of improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
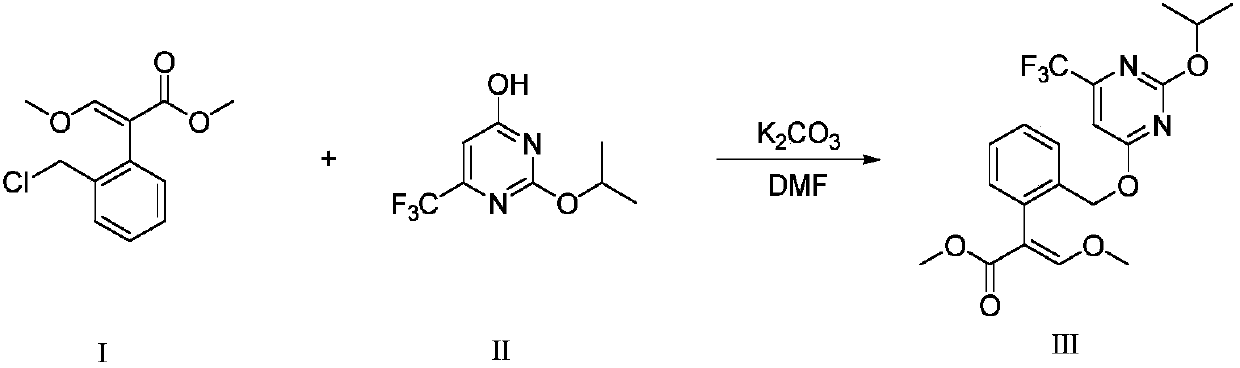
[0030] (1) Add 20 g (0.083 moL) of (E)-2-(2'-chloromethyl)phenyl-3-methoxymethyl acrylate, 2-iso Propoxy-4-hydroxy-6-trifluoromethylpyrimidine 22.15g (0.100moL), potassium carbonate 6.32g (0.046moL) and DMF189.6g, stirred and dissolved, heated to 120°C, TLC followed the reaction to (E) - Methyl 2-(2'-chloromethyl)phenyl-3-methoxyacrylate is completed.
[0031] (2) Pour the feed liquid into 200g of 0.5% sodium hydroxide aqueous solution after cooling, stir and separate out viscous matter, extract 80g × 3 times with industrial ethyl acetate, wash with water, dry, and concentrate under reduced pressure until dry to obtain orange-red oily 37g of the product (i.e. pyrimiden crude product) was used for refining.
[0032] (3) Add 140g of ethanol to the crude product of pyrimidone, heat to dissolve and transfer it into a 500mL three-neck flask with mechanical stirring and a thermometer, stir and heat to reflux, then suction filter while it is hot, and re-transfer the filtrate into a ...
Embodiment 2
[0034] (1) Add 90 g (0.37 moL) of (E)-2-(2'-chloromethyl)phenyl-3-methoxymethyl acrylate to a 3000 mL three-necked flask equipped with a mechanical stirrer and a thermometer, 2-iso Propoxy-4-hydroxyl-6-trifluoromethylpyrimidine 99g (0.45moL), potassium carbonate 54g (0.39moL) and DMF900g, stirred and dissolved, heated to 120 ° C, TLC followed the reaction to (E)-2-( Methyl 2'-chloromethyl)phenyl-3-methoxyacrylate is completed.
[0035] (2) Pour the feed liquid into 900g of 0.5% aqueous sodium hydroxide solution after cooling, stir and separate out viscous matter, extract 360g × 3 times with industrial ethyl acetate, wash with water, dry, and concentrate under reduced pressure to dryness to obtain orange-red oily 170g of the product (i.e. the crude product of pyrimidin) was all used for refining.
[0036] (3) Add 810g of ethanol to the crude product of pyrimidin, heat to dissolve and then transfer it into a 3000mL three-necked flask with mechanical stirring and a thermometer. ...
Embodiment 3
[0038] (1) Add 720 g (2.99 moL) of (E)-2-(2'-chloromethyl)phenyl-3-methoxymethyl acrylate to a 10L three-necked flask with mechanical stirring and a thermometer, 2- 792g (3.56moL) of isopropoxy-4-hydroxyl-6-trifluoromethylpyrimidine, 216g (1.56moL) of potassium carbonate and 7.2kg of DMF, stirred and dissolved, heated to 135°C, TLC tracked the reaction to (E)- Methyl 2-(2'-chloromethyl)phenyl-3-methoxyacrylate is completed.
[0039] (2) feed liquid is poured in the 0.5% sodium hydroxide aqueous solution of 7.2kg after cooling, stirs and separates out viscous thing, industrial ethyl acetate extracts 2.8kg * 3 times, washes with water, dries, and concentrates under reduced pressure to dryness to obtain orange 1.4kg of red oily substance (ie pyrimaben crude product) was used for refining.
[0040] (3) Add 6.5kg of ethanol to the crude pyrimidofen oil, heat to dissolve and transfer it into a 10L three-necked flask with mechanical stirring and a thermometer, stir and heat to reflu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com