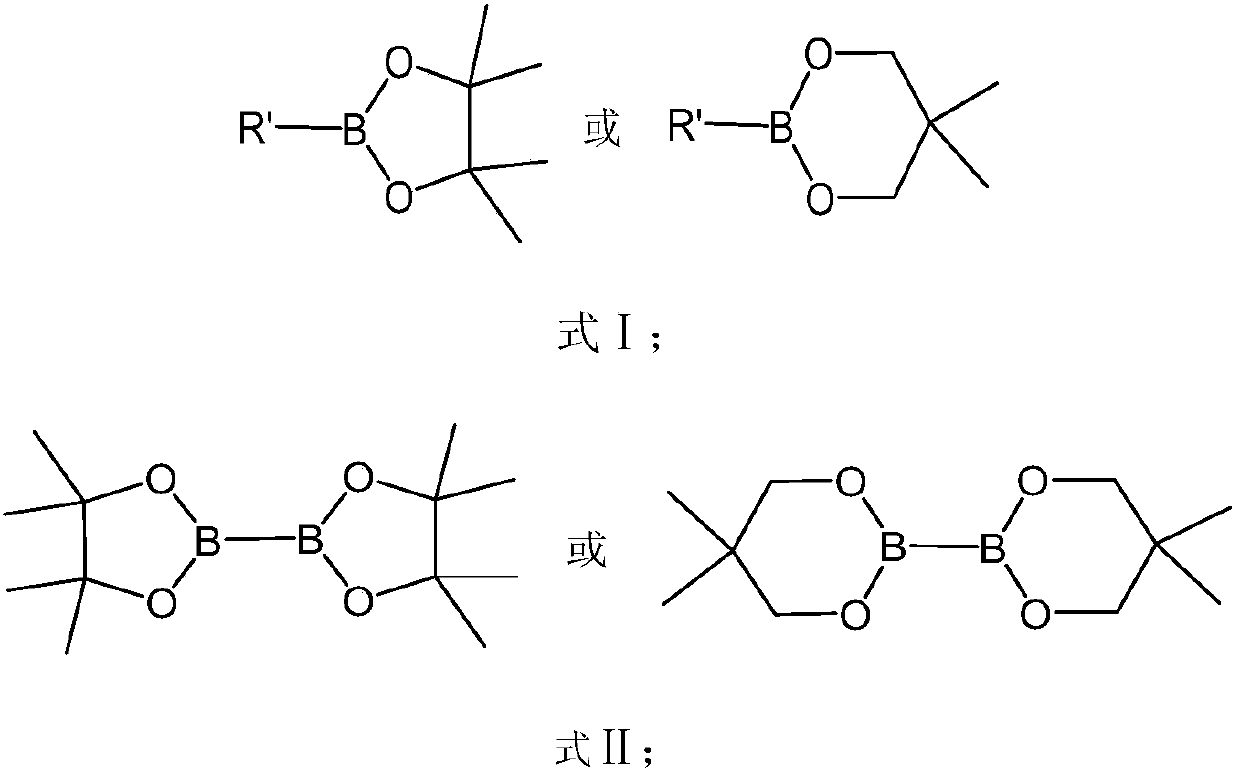
Method for preparing aryl boronate at room temperature
An aryl borate, room temperature technology, applied in the field of preparing aryl borate at room temperature, can solve the problems of unsuitable large-scale preparation, harsh reaction conditions, large catalyst dosage, etc., and achieves a wide range of applications, mild reaction conditions, and compatibility. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
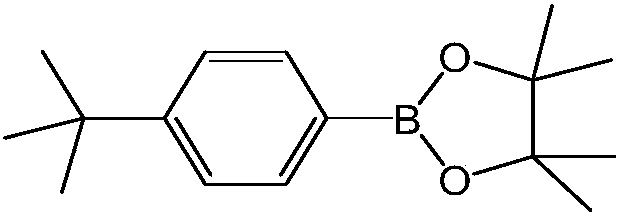
[0020] Embodiment 1: the synthesis of 4-tert-butylphenylboronic acid pinacol ester
[0021]
[0022] Method (1): take 4-tert-butylchlorobenzene as raw material synthesis: add K in the reaction flask successively 3 PO 4 ·7H 2 O (3.0g, 8.85mmol), bis-pinacol borate (749mg, 2.95mmol), catalyst chlorine (2-dicyclohexylphosphino-2',4',6'-tri-isopropyl- 1,1'-biphenyl)(2'-amino-1,1'-biphenyl-2-yl)palladium(II) (12mg, 0.015mmol) and the ligand 2-dicyclohexylphosphine-2', 4′,6′-Triisopropylbiphenyl (4mg, 0.008mmol), then add EtOH (6mL) and stir well, add p-tert-butylchlorobenzene (0.5mL, 2.95mmol), react at room temperature for 0.5h, wait After the reaction, the reaction solution was diluted with ethyl acetate (2 mL), filtered through diatomaceous earth to remove insoluble matter, ethyl acetate (6 mL) was washed three times, the filtrate was combined, concentrated under reduced pressure to remove the solvent, and filtered with silica gel (200 ~ 300 mesh) was separated by column ...
Embodiment 2
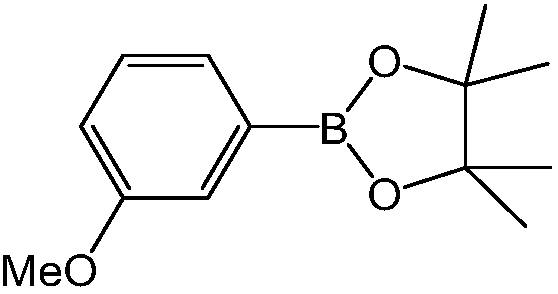
[0024] Embodiment 2: the synthesis of 3-methoxyphenylboronic acid pinacol ester
[0025]
[0026] Method (1): Taking 3-methoxychlorobenzene as raw material synthesis: the method (1) of reference example 1, the difference is that the p-tert-butylchlorobenzene used uses equimolar 3-methoxychlorobenzene Benzene was replaced, other steps were the same as the method (1) of Example 1, and the obtained white solid was identified as 3-methoxyphenylboronic acid pinacol ester by NMR spectrum, and the yield was 92%. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 7.41 (m, 1H), 7.33 (d, J = 3.5Hz, 1H), 7.29 (s, 1H), 7.01 (ddd, J 1 =8.2Hz,J 2 =2.8Hz,J 3 =1.2Hz,1H),3.84(s,3H),1.35(s,12H); 13 C NMR (75MHz, CDCl 3 )δ (ppm): 159.2, 129.1, 127.4, 118.9, 118.1, 84.2, 55.4, 25.1.
[0027] Method (2): taking 3-methoxy bromobenzene as raw material synthesis: with reference to the method (1) of this embodiment, the difference is that the 3-methoxy chlorobenzene used uses equimolar 3-methoxy bromide Benz...
Embodiment 3
[0028] Embodiment 3: the synthesis of 4-hydroxymethylphenylboronic acid pinacol ester
[0029]
[0030] Method (1): taking 4-chlorobenzyl alcohol as raw material synthesis: the method (1) of reference example 1, the difference is that used p-tert-butylchlorobenzene is replaced with equimolar 4-chlorobenzyl alcohol, and the reaction The time was 2 hours, and other steps were the same as the method (1) of Example 1, and the obtained white solid was identified as 4-hydroxymethylphenylboronic acid pinacol ester by NMR spectrum, and the yield was 96%. 1 H NMR (300MHz, CDCl 3)δ (ppm): 7.78 (dd, J 1 =12.8Hz,J 2 =8.1Hz,2H),7.38(dd,J 1 =12.8Hz,J 2 =8.1Hz, 2H), 4.70(s, 2H), 1.35(s, 12H); 13 C NMR (75MHz, CD 3 OD) 146.2, 136.0, 127.2, 85.2, 65.2, 25.3.
[0031] Method (2): taking 4-iodobenzyl alcohol as raw material synthesis: with reference to the method (1) of this embodiment, the difference is that the 4-chlorobenzyl alcohol used is replaced with equimolar 4-iodobenzyl alcoh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com