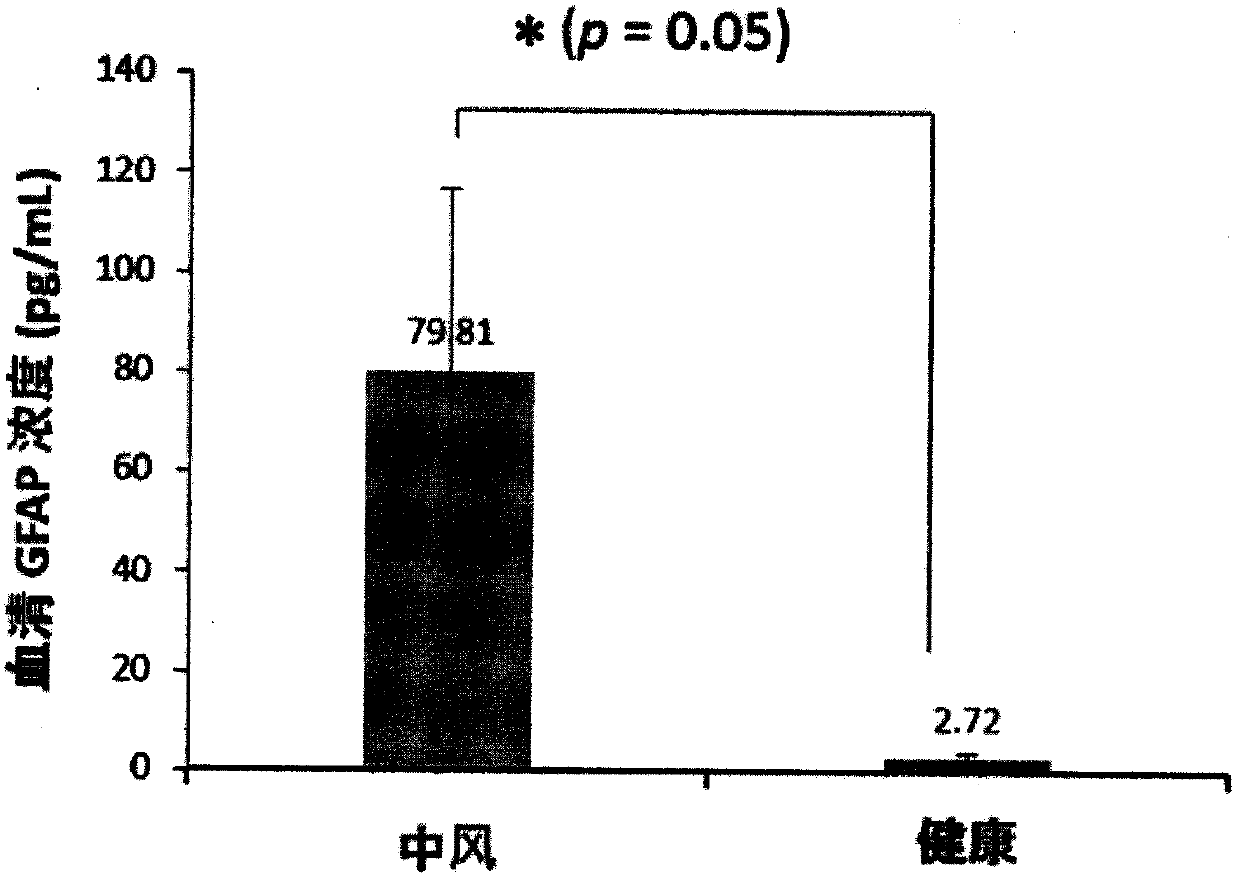
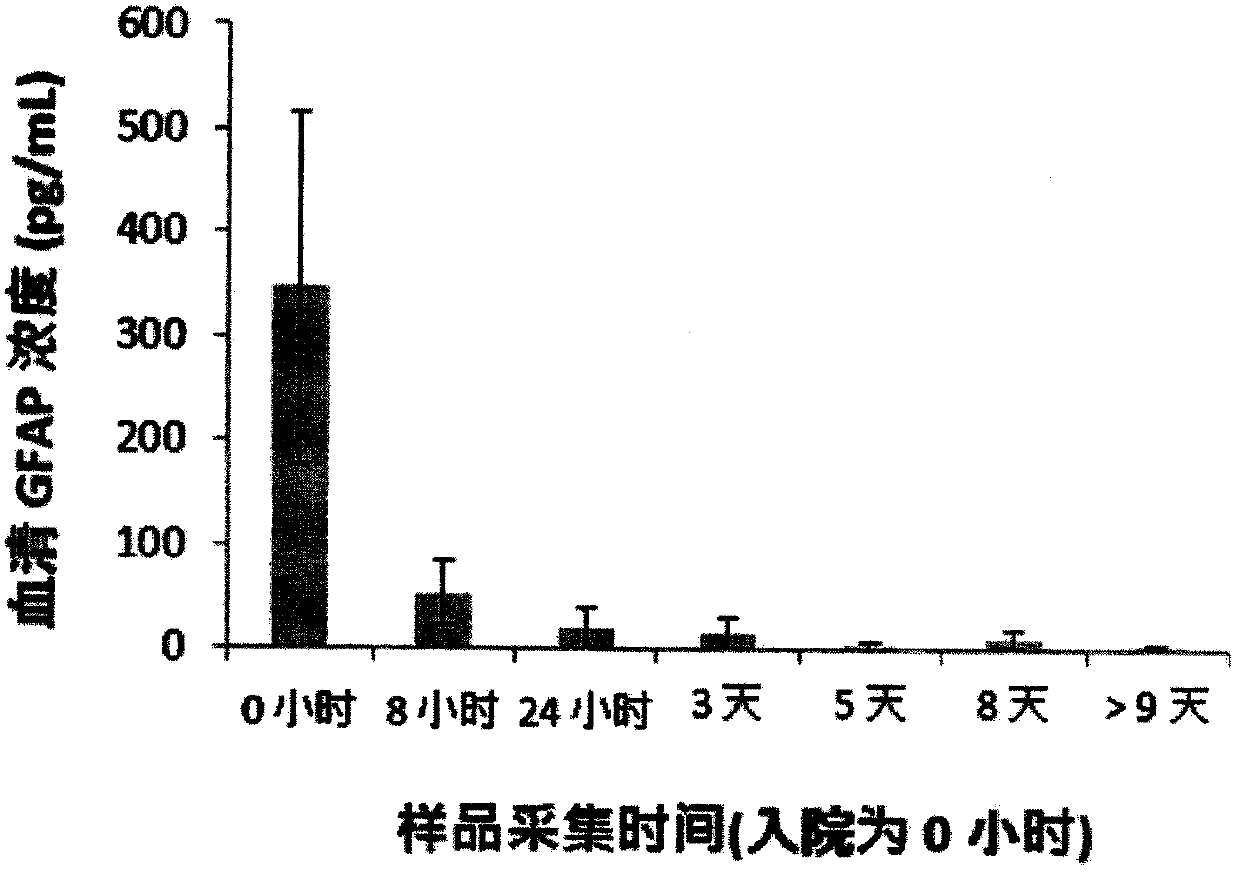
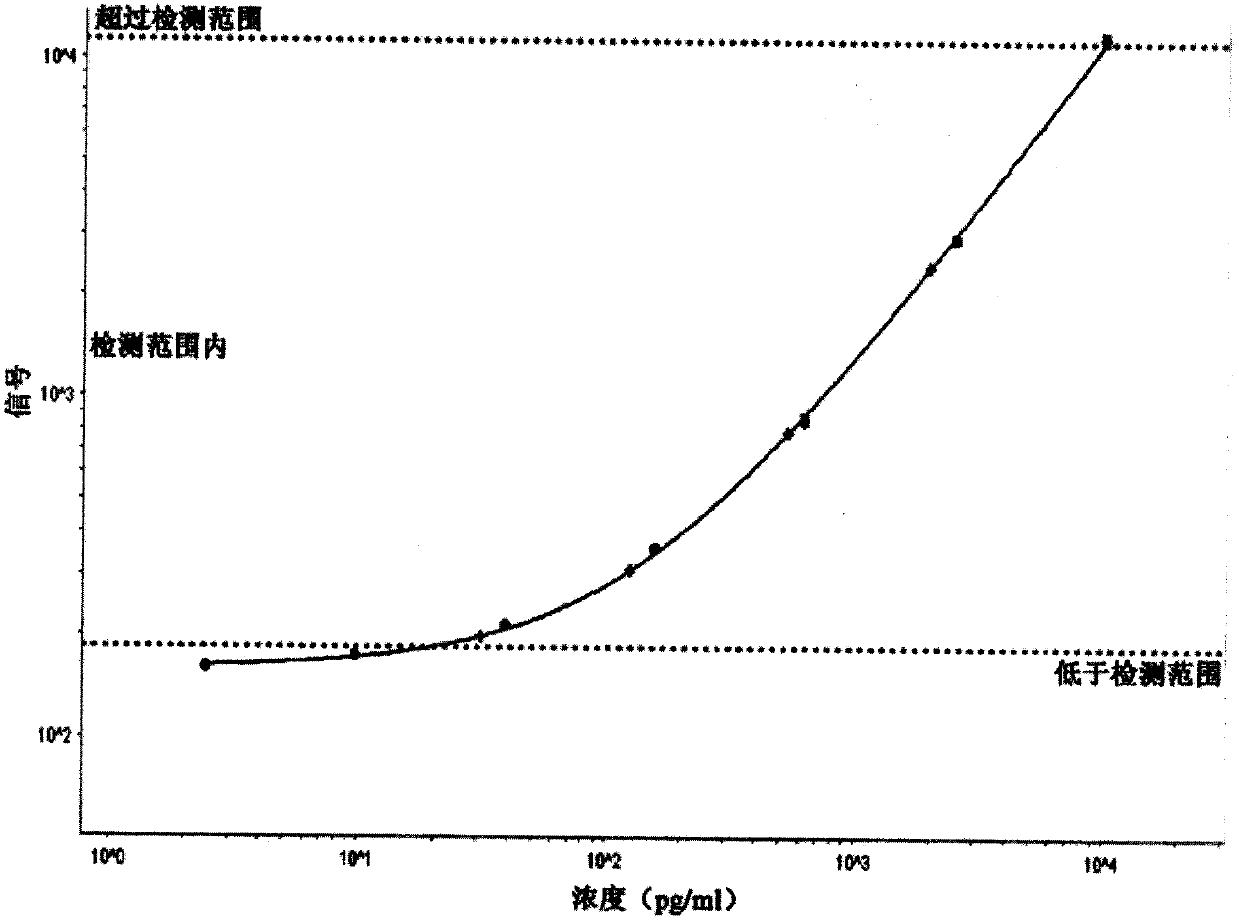
Luminescent ELISA in vitro diagnosis reagent kit for cerebral apoplexy and in vitro test equipment
An in vitro diagnosis and kit technology, applied in the field of biomedical testing, can solve problems such as the difficulty in distinguishing hemorrhagic stroke from ischemic stroke, the inability to judge the treatment effect of damaged cell types, and the low level of stroke diagnosis, so as to improve the accuracy and reproducibility, avoiding spinal taps, and reducing testing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] Preparation of serum to be tested
[0075] Extract blood from cerebral apoplexy patients intravenously; place at room temperature for 1-2 hours to allow natural layering and separation of blood cells and serum; mix evenly the serum with the aqueous solution for serum sample stabilization at a ratio of 2:1-1:10, and set aside.
[0076] ELISA
[0077] Prepare 48 or 96 well microtiter plates (elisa plates). Adsorb the GFAP monoclonal antibody in the small hole of the microtiter plate, and wash once with PBS; add the diluted stroke patient serum prepared above to the small hole of the microtiter plate; then wash off the unbound excess antibody; Then, a detection antibody that has been coupled to a luminescent group in advance is added to the small well, and the coupling detection antibody is preferably an animal-derived antiserum (ie, a polyclonal antibody). Then wash the wells of the microtiter plate 3-4 times.
[0078] Antigen content determination
[0079] After wash...
preparation example 1
[0086] Preparation of Aqueous Solution 1 for Body Fluid Sample Stabilization
[0087] Prepare 50 mL of sterile distilled water, add 10 mL of normal human serum (BioreclamationIVT, USA), 1 g of bovine serum albumin (Sigma, USA), 2 mL of Tween 20 (Sigma-Aldrich, USA), and a final concentration of 0.05 mM urea (Sigma- Aldrich, USA), sodium chloride (Sigma-Aldrich, USA) at a final concentration of 110 mM, potassium chloride (Sigma-Aldrich, USA) at a final concentration of 2.0 mM, and Tris base (Sigma-Aldrich , USA), and finally add the remaining amount of sterile distilled water to mix evenly and make the volume to 100mL.
preparation example 2
[0089] Preparation of aqueous solution 2 for stabilization of body fluid samples
[0090] Prepare 50 mL of sterile distilled water, add 20 mL of normal human serum (BioreclamationIVT, USA), 2 g of bovine serum albumin (Sigma, USA), 2 mL of Tween 20 (Sigma-Aldrich, USA), and a final concentration of 0.08 mM urea (Sigma- Aldrich, USA), a final concentration of 125mM sodium chloride (Sigma-Aldrich, USA), a final concentration of 2.3mM potassium chloride (Sigma-Aldrich, USA), a final concentration of 15mM Tris base (Sigma-Aldrich, USA), and finally add the remaining amount of sterile distilled water to mix evenly and make the volume to 100mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com