Preparation process of fluorine-free superhydrophobic surface
A technology of super-hydrophobic surface and preparation process, which is applied in the field of super-hydrophobic surface preparation on metal aluminum by using rosin instead of fluoride, which can solve the problems of loss of super-hydrophobic properties, easy wear, shedding, unfavorable industrialization, etc., and achieve the goal of suppressing freezing point The effect of formation, small rolling angle and cheap chemical reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
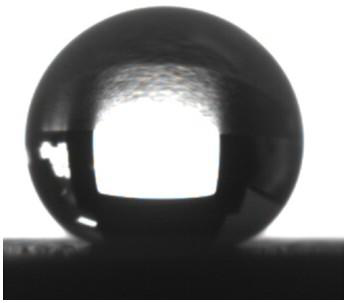
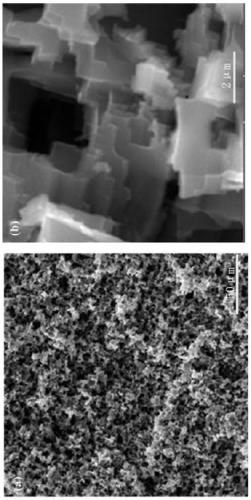
[0028] The cut aluminum sheet was polished evenly with 400 and 1200 mesh sandpaper, and each sample was ultrasonically cleaned with acetone, ethanol, and deionized water for 5 minutes and then dried. Prepare the concentrated hydrochloric acid into 1M dilute hydrochloric acid, add a certain mass of copper chloride particles, and stir evenly to form a 0.2M copper chloride hydrochloric acid solution. Put the dried sample into the solution and react for 4 minutes, then take it out, wash off the copper attached to the surface, put it into the solution and continue to react for 4 minutes, take the sample out, ultrasonically clean it and dry it, its surface appearance is as follows: figure 1 shown. Then a certain mass of rosin was dissolved in absolute ethanol to prepare a rosin alcohol solution with a mass fraction of 1%. After the dried sample was immersed in the rosin alcohol solution, it was quickly taken out, placed on filter paper to blot the excess solution, and after natural...
Embodiment 2
[0030] The cut aluminum sheet was polished evenly with 400 and 1200 mesh sandpaper, and each sample was ultrasonically cleaned with acetone, ethanol, and deionized water for 5 minutes and then dried. Prepare the concentrated hydrochloric acid into 2M dilute hydrochloric acid, add a certain mass of copper chloride particles, and stir evenly to form a 0.2M copper chloride hydrochloric acid solution. The dried sample was put into the solution and reacted for 4 minutes, then taken out, ultrasonically cleaned and dried. Then a certain mass of rosin was dissolved in absolute ethanol to prepare a rosin alcohol solution with a mass fraction of 2.5%. The solution was dropped into the center of the dry sample with a disposable needle. Since the sample is super-affinity to alcohol, the solution will smear quickly, and a super-hydrophobic surface will be obtained after drying at 150°C.
Embodiment 3
[0032] The cut Al7075 alloy was polished evenly with 400 and 1200 mesh sandpaper, and the samples were ultrasonically cleaned with acetone, ethanol, and deionized water for 5 minutes and then dried. Prepare the concentrated hydrochloric acid into 2M dilute hydrochloric acid, add a certain mass of copper chloride particles, and stir evenly to form a 0.2M copper chloride hydrochloric acid solution. The dried sample was put into the solution and reacted for 4 minutes, then taken out, ultrasonically cleaned and dried. Then a certain mass of rosin was dissolved in absolute ethanol to prepare a rosin alcohol solution with a mass fraction of 4%. The solution was dropped into the center of the dry sample with a disposable needle. Since the sample is super-affinity to alcohol, the solution will smear quickly, and a super-hydrophobic surface will be obtained after drying at 150°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com