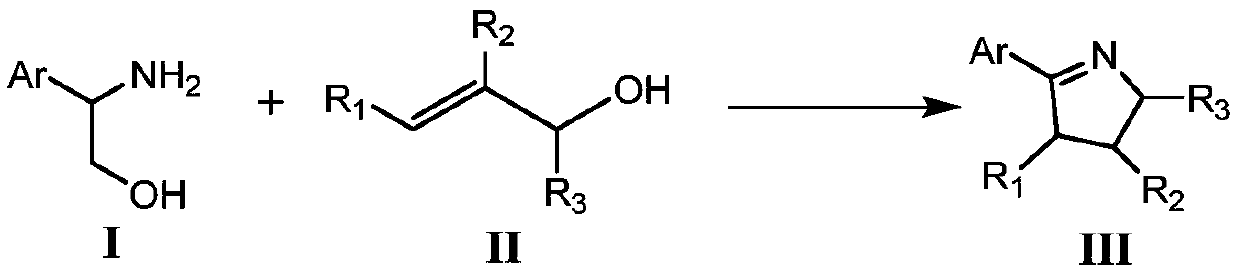
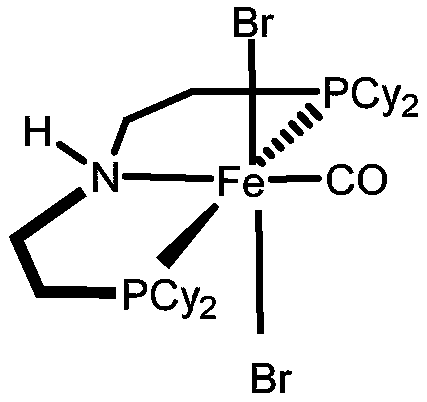
Method for synthesizing pyrroline compounds through iron-catalyzed amino alcohol and enol
An iron-catalyzed aminoalcohol, a technology for catalyzing aminoalcohols, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., to achieve mild reaction conditions, good applicability, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 2-(4'-methylphenyl)-1-pyrroline with the following structural formula
[0028]
[0029] Mix 24μL (0.0225mmol) sodium triethylborate with 5.3mg (0.0075mmol) [(Cy-PN H P)Fe(CO)(Br) 2 ], 1.5mL toluene was added to the reaction tube, mixed and stirred for 10 minutes, then 37.75mg (0.25mmol) 2-amino-1-(4-methylphenyl) ethanol, 34μL (0.5mmol) allyl alcohol, 48mg (0.375mmol) Potassium tert-butoxide, stirred and reacted at 120°C for 12 hours under the protection of argon, cooled to room temperature after the reaction, transferred with dichloromethane, dichloromethane and toluene were removed by distillation under reduced pressure, and purified with petroleum ether and ethyl acetate The volume ratio of the ester is 5:1 mixed solution as the eluent, and the product is separated by flash column chromatography to obtain 2-(4'-methylphenyl)-1-pyrroline with a yield of 73%, characterization data for: 1 H NMR (400MHz, CDCl 3 ):δ(ppm)7.74-7.72(d,2H),7.26-7.20(m,2H...
Embodiment 2
[0031] Preparation of 2-(benzo[d][1,3]dioxolyl)-1-pyrroline with the following structural formula
[0032]
[0033] In this example, the 2-amino-1-(4-methyl phenyl) ethanol, other steps are identical with embodiment 1, obtain 2-(benzo[d][1,3]dioxolyl)-1-pyrroline, its productive rate is 51%, characterization The data is: 1 H NMR (400MHz, CDCl 3 ): δ(ppm)7.43-7.42(d,1H),7.29-7.27(d,2H),6.83-6.81(d,1H),5.99(s,2H),4.04-4.01(t,2H),2.91 -2.86(t,2H),2.06-1.98(m,2H); 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 172.5, 149.5, 148.0, 129.4, 122.7, 108.0, 107.5, 101.5, 61.4, 35.1, 22.9; HRMS (ESI + ) m / z C 10 h 11 N[M+H] + : The experimental value is 190.0868, and the theoretical value is 190.0859.
Embodiment 3
[0035] Preparation of 2-(4'-chlorophenyl)-1-pyrroline with the following structural formula
[0036]
[0037] In this example, the 2-amino-1-(4-methylphenyl)ethanol in Example 1 is replaced with equimolar 2-amino-1-(4-chlorophenyl)ethanol, and the other steps are the same as in Example 1 Identical, obtain 2-(4'-chlorophenyl)-1-pyrroline, its yield rate is 67%, and characteristic data is: 1 H NMR (400MHz, CDCl 3 ):δ(ppm)7.77-7.75(d,2H),7.38-7.36(m,2H),4.07-4.03(m,2H),2.93-2.88(m,2H),2.07-2.00(m,2H) ; 13 C NMR (100MHz, CDCl 3 ): δ (ppm) 172.3, 136.4, 133.2, 129.0, 128.8, 61.7, 35.0, 22.8; HRMS (ESI + )m / zC 10 h 11 N[M+H] + : The experimental value is 180.0588, and the theoretical value is 180.0580.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com