A fluorescent probe capable of rapidly detecting bisulfite ions and its preparation method and application
A technology of bisulfite and fluorescent probes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of long reaction time, fluorescence color change, low sensitivity, etc., and achieve high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of 3-(4″-formyl-1′, 1″-biphenyl-4′-carbonyl)nopinone-based fluoroboron complex, the reaction formula:
[0036]
[0037] Specific steps are as follows:
[0038] 1, the preparation of 3-(4'-bromobenzoyl) nopinone:
[0039] Add 0.06 mol of sodium hydride into a three-neck flask equipped with a stirrer, thermometer and reflux condenser, add 8 mL of ethylene glycol dimethyl ether under nitrogen protection, and dissolve 0.02 mol of nopinone in 9 mL of ethylene glycol dimethyl ether Slowly add into the flask, heat to reflux for 0.5h, then slowly add 0.024mol methyl 4-bromobenzoate dissolved in 9mL of ethylene glycol dimethyl ether into the flask for reflux reaction for 7-8h, track the reaction progress with thin-layer chromatography. After the reaction, the reaction solution was cooled in an ice bath, slowly added 15 mL of distilled water to hydrolyze sodium hydride, extracted 3 times with 45 mL of ethyl acetate, the combined organic phase was washed...
Embodiment 2
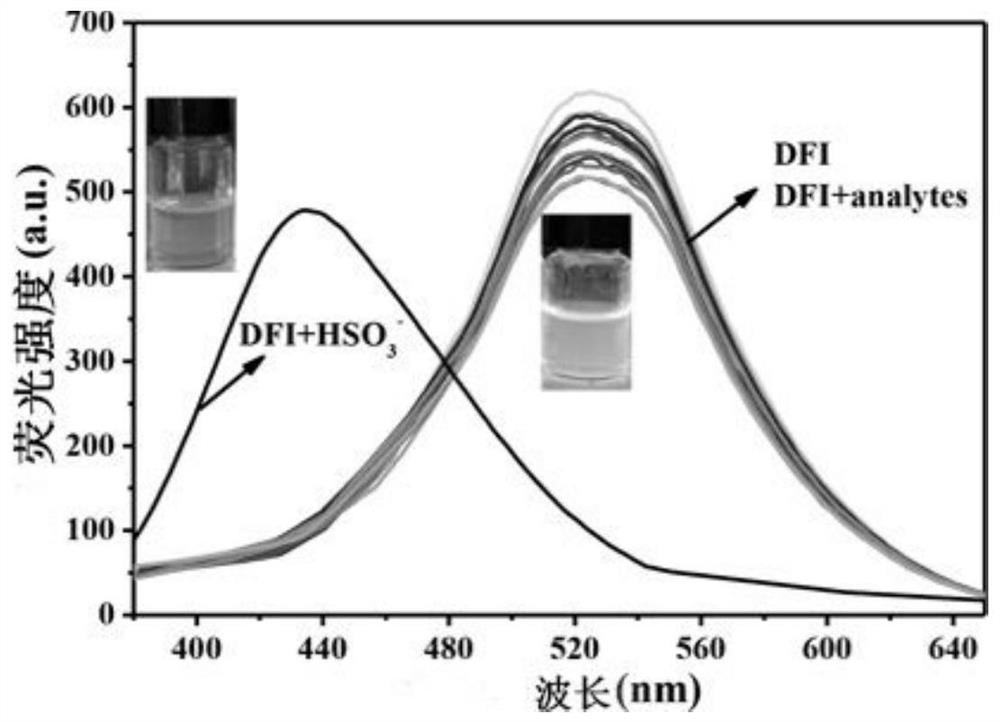
[0045] 3-(4″-formyl-1′, 1″-biphenyl-4′-carbonyl)nopinone-based fluoroboron complex and HSO 3 - , SO 3 2- , HS - , F - , Cl - , Br - , I - , AcO - , SCN - , NO 2 - , NO 3 - , HSO 4 - , H 2 PO 4 - , HPO 4 2- , SO 4 2- , CO 3 2- 16 different anions are dissolved in 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer solution (20mM, pH=7.2, 7 / 3 (v / v) ethanol / water), and the concentration is 1.0 ×10 -6 M probe solution and a concentration of 1.0 x 10 -5 Solutions of 16 different anions of M. Under the condition that the excitation wavelength is 360am, the fluorescence intensity changes in the presence of different anions are measured, such as figure 1 shown. The results showed that in HSO 3 - In the presence of ions, the maximum emission wavelength of the solution blue shifts from 520nm to 435nm. The fluorescent color of the solution changed from green to blue under the ultraviolet light, indicating that the compound is sensitive to HSO 3 - i...
Embodiment 3
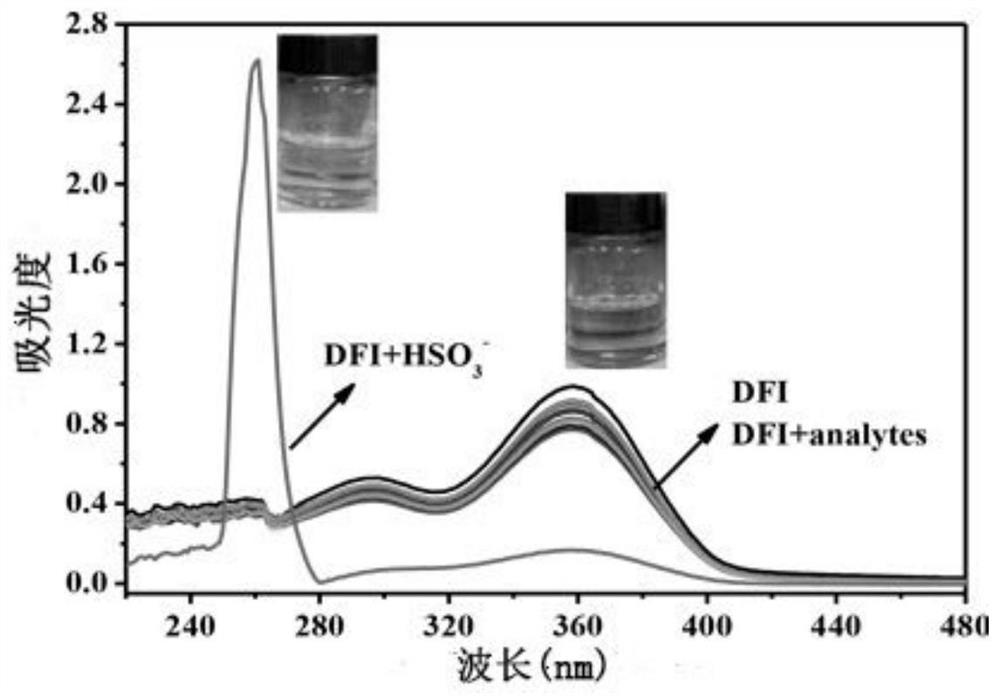
[0047] Dissolve the solid 3-(4″-formyl-1′,1″-biphenyl-4′-carbonyl)nopinonylfluoroboron complex and 16 different anions in 4-hydroxyethylpiperazine In ethanesulfonic acid (HEPES) buffer solution (20mM, pH=7.2, 7 / 3 (v / v) ethanol / water), the concentration is 1.0×10 -6 M probe solution and a concentration of 1.0 x 10 -5 Solutions of 16 different anions of M. Measured HSO 3 - , SO 3 2- , HS - , F - , Cl - , Br - , I - , AcO - , SCN - , NO 2 - , NO 3- , HSO 4 - , H 2 PO 4 - , HPO 4 2- , SO 4 2- , CO 3 2- The ultraviolet absorption spectrum of the probe solution under the presence of 16 different anions, the results are as follows figure 2 shown. Probe solution in HSO 3 - In the presence of cyanide, the maximum absorption peak of the ultraviolet absorption spectrum is blue-shifted from 360nm to 260nm. It shows that the compound can be used as a color probe for HSO 3 - to test.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com