A kind of synthetic method of 2-substituted indole derivatives
A synthesis method, C2-C6 technology, applied in the field of synthesis of 2-substituted indole derivatives, can solve the problems of reducing the flexibility of reconversion synthesis building blocks, uneconomical large-scale synthesis, and difficult post-processing, etc., and achieves easy industrialization The effect of less production and waste discharge and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
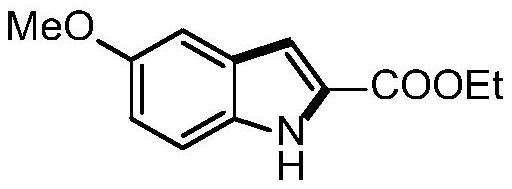
[0023] Example 1 Synthesis of ethyl 5-methoxyl-1H-indole-2-carboxylate
[0024]
[0025] a): Take a 25mL Schlenk reaction tube, add p-methoxyaniline 49mg, palladium acetate 9mg and Molecular sieve 80mg, inject ethyl 2-oxopropionate 93mg, acetic acid 96mg and dimethyl sulfoxide 2mL, connect a 200mL oxygen balloon, stir at 70°C for 18 hours. After the reaction was completed, 15 mL of ethyl acetate was added to dilute the reaction solution, filtered and washed twice with 10 mL of brine, the organic phase was separated, the aqueous phase was extracted once with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 5-methoxy- 75 mg of pure ethyl indole-2-carboxylate, yield 86%.
[0026] b): Take a 25mL Schlenk reaction tube, add p-methoxyaniline 49mg, palladium acetate 9mg and Molecular sieve 80mg, inject ethyl 2-oxopropionate 93mg, acetic acid 96mg and dimethyl sulfoxide 2mL, and stir at 70°C for 18 hours under normal air pressure...
Embodiment 2
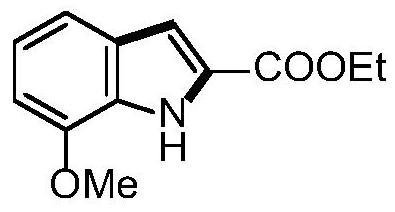
[0047] Example 2 Synthesis of ethyl 7-methoxyl-1H-indole-2-carboxylate
[0048]
[0049] Take a 25mL Schlenk reaction tube, add o-methoxyaniline 49mg, palladium acetate 9mg and Molecular sieve 80mg, inject ethyl 2-oxopropionate 93mg, acetic acid 96mg and dimethyl sulfoxide 2mL, connect a 200mL oxygen balloon, stir at 70°C for 18 hours. After the reaction was completed, 15 mL of ethyl acetate was added to dilute the reaction solution, filtered and washed twice with 10 mL of brine, the organic phase was separated, the aqueous phase was extracted once with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 74 mg of the pure product of the target product. Yield 85%.
[0050] 1 H NMR (400MHz, Chloroform-d) δ: 9.17 (brs, 1H), 7.26 (d, J = 8.1, 1H), 7.19 (d, J = 2.2, 1H), 7.04 (t, J = 7.9, 1H) ,6.69(d,J=7.6,1H),4.39(q,J=7.1,2H),3.93(s,3H),1.39(t,J=7.1,3H); 13 C NMR (101MHz, Chloroform-d) δ: 161.91, 146.57, 128.74, 128.18, 127.34,...
Embodiment 3
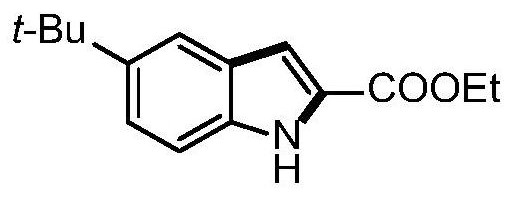
[0051] Example 3 Synthesis of ethyl 5-tert-butyl-1H-indole-2-carboxylate
[0052]
[0053] Take a 25mL Schlenk reaction tube, add p-tert-butylaniline 60mg, palladium acetate 9mg and Molecular sieve 80mg, inject ethyl 2-oxopropionate 93mg, acetic acid 96mg and dimethyl sulfoxide 2mL, connect a 200mL oxygen balloon, stir at 70°C for 18 hours. After the reaction was completed, 15 mL of ethyl acetate was added to dilute the reaction solution, filtered and washed twice with 10 mL of brine, the organic phase was separated, the aqueous phase was extracted once with ethyl acetate, the organic phases were combined, and separated by column chromatography to obtain 74 mg of the pure product of the target product. Yield 76%.
[0054] 1 H NMR (400MHz, Chloroform-d) δ: 9.22 (brs, 1H), 7.67–7.63 (m, 1H), 7.41 (dd, J = 8.8, 1.8, 1H), 7.35 (dt, J = 8.8, 0.8, 1H), 7.19(dd, J=2.1, 0.9, 1H), 4.41(q, J=7.1, 2H), 1.41(t, J=7.1, 3H), 1.37(s, 9H); 13 C NMR (101MHz, Chloroform-d) δ: 162.44, 143....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com