Crystal form of linagliptin and preparation method thereof
A crystal form and molecular crystallization technology, applied in the fields of active ingredients of heterocyclic compounds, metabolic diseases, organic chemistry, etc., can solve the problems of linagliptin's poor crystal form stability, incapable of large-scale production, and safety , to achieve the effect of good drug prospects, low hygroscopicity and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of linagliptin crystal form.
[0057] Add 10 g of linagliptin solid to 40 mL of methyl ethyl ketone to form a suspension, and stir at a stirring speed of 300 r / min. Raise the temperature to 30°C at a heating rate of 3°C / min to obtain a linagliptin clear liquid, add 2ml of purified water to the above clear liquid at a constant temperature, then cool down to 20°C at a cooling rate of 0.5°C / min, and grow crystals at a constant temperature for 1.5h . Suction filtration, and dry the obtained wet crystalline product at 40°C and a vacuum of 0.1Mpa for 10h to obtain 9.00g of crystalline solid. The purity was 99.6%, and the yield was 90.0%.
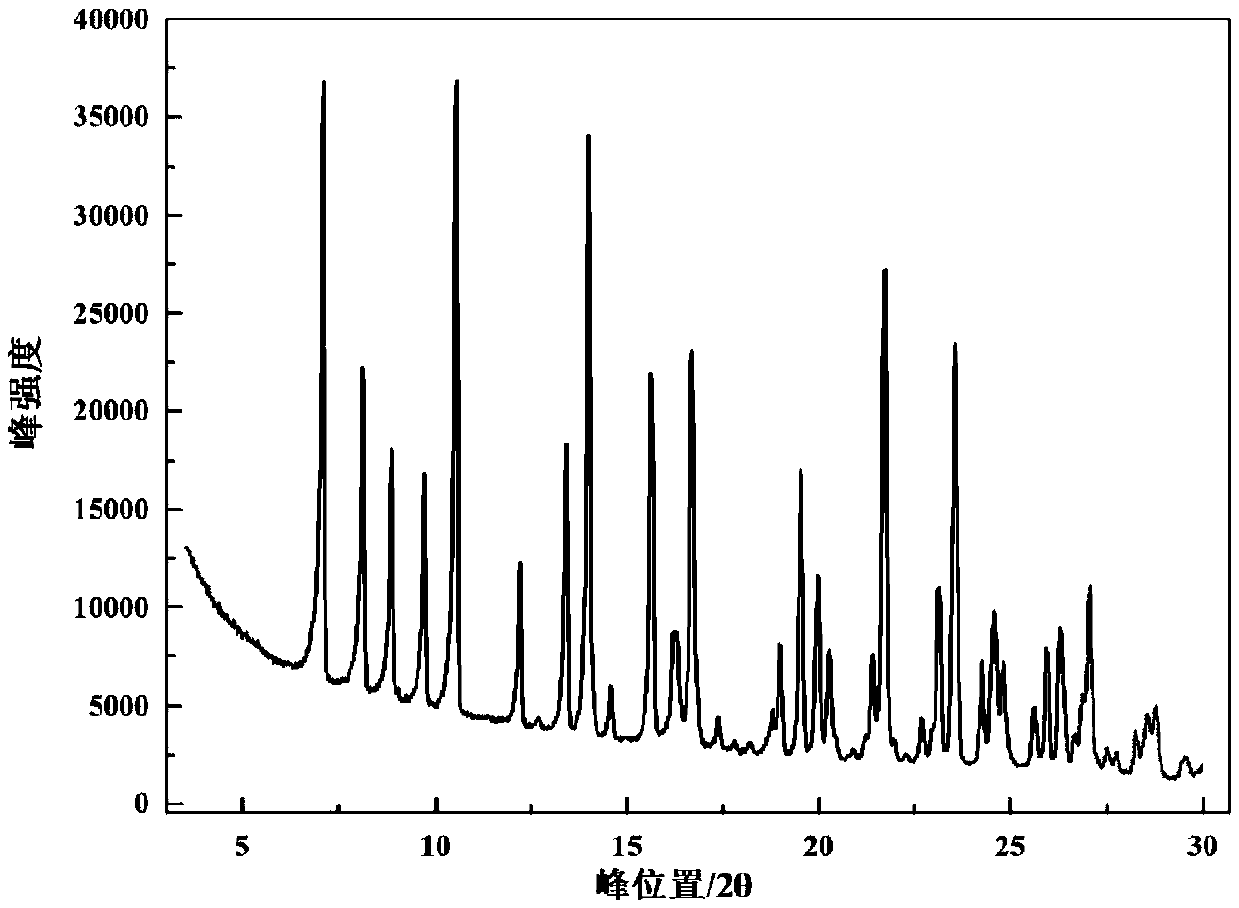
[0058] The characterization data of the crystal form determined by XRD are shown in Table 1, and the XRD spectrum of its sample is shown in Table 1. figure 1 shown.
[0059] Table 1 XRD analysis data of the crystal form of the application
[0060]
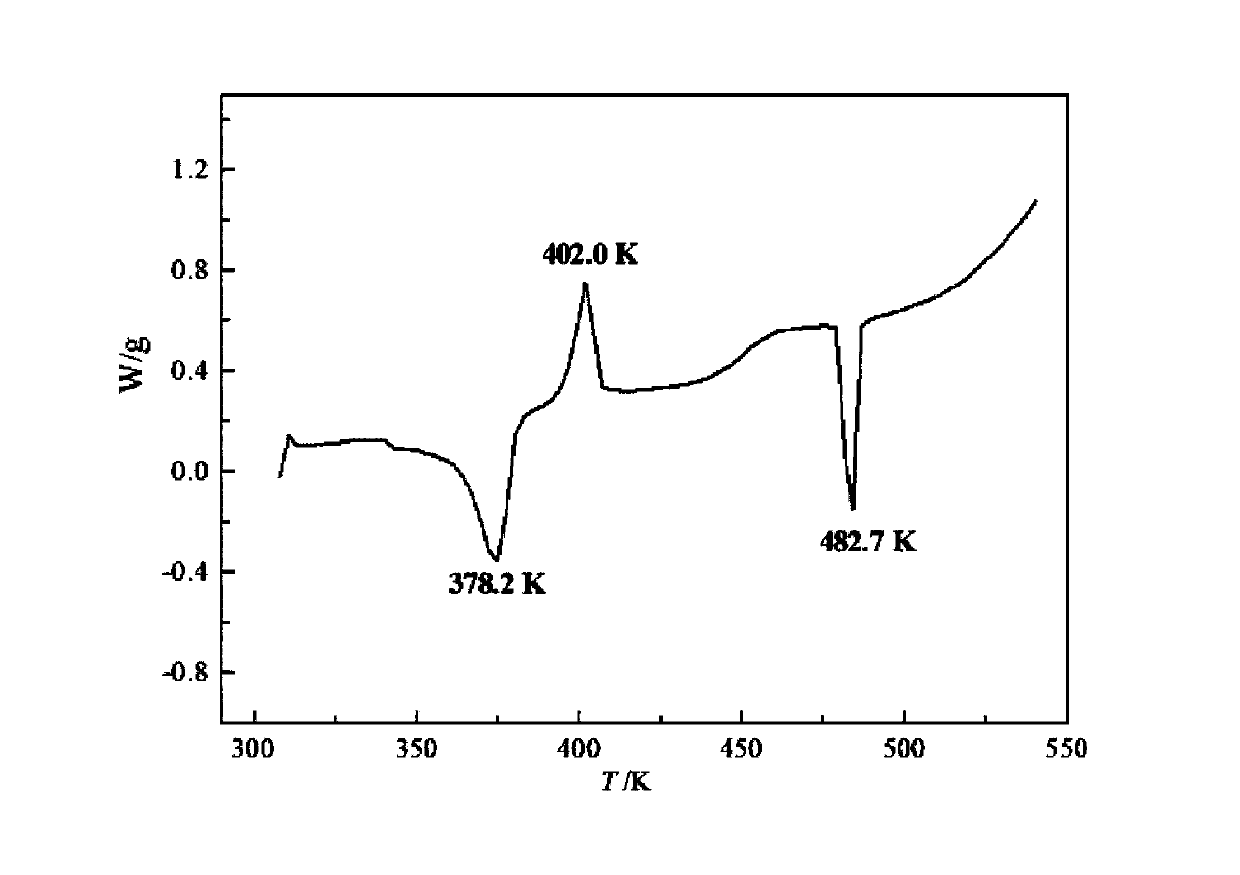
[0061] The results of DSC analysis show that there are strong...
Embodiment 2
[0067] Embodiment 2: Preparation of linagliptin crystal form
[0068] Add 10 g of linagliptin solid into 40 mL of a mixed solvent of methanol and acetone (volume ratio = 1:1) and stir at a stirring speed of 200 r / min. Raise the temperature to 70°C at a heating rate of 2°C / min to obtain a linagliptin clarified liquid, add 1.6mL of purified water to the above clear liquid at a constant temperature, then cool down to 0°C at a cooling rate of 0.5°C / min, and grow crystals at a constant temperature of 0.5°C. h. Suction filtration, and dry the obtained wet crystalline product at 55°C and a vacuum of 0.08Mpa for 2h to obtain 9.12g of crystalline solid. The purity was 99.7%, and the yield was 91.2%.
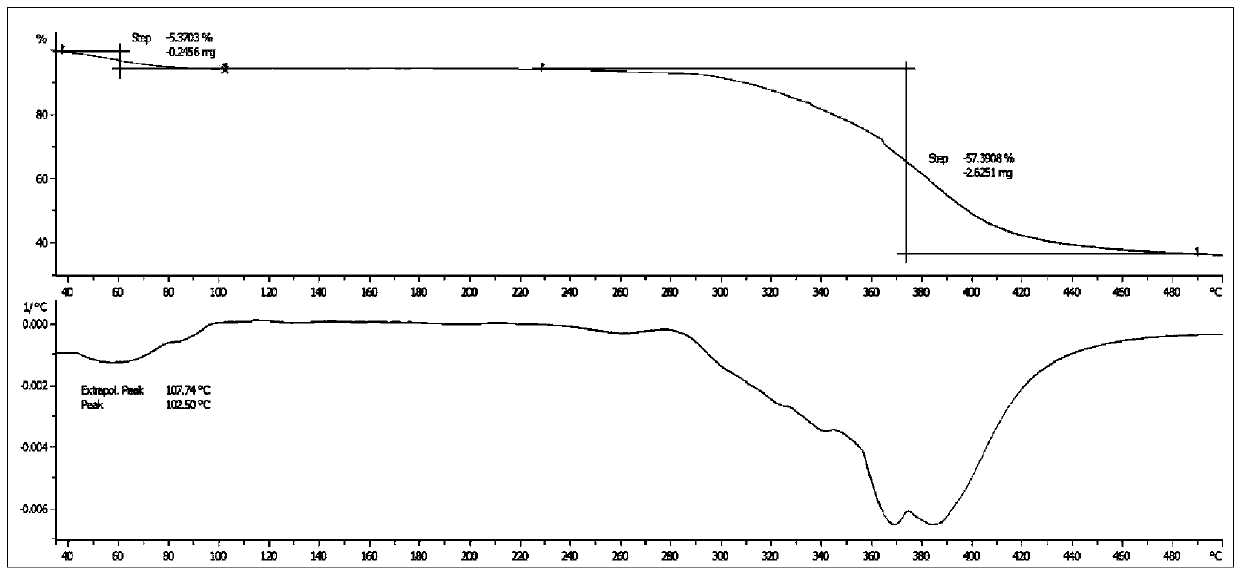
[0069] After determination, its XRD pattern and figure 1 Basically the same, its DSC spectrum and figure 2 Basically the same, according to the KF moisture test, its water content is about 6.1%, and its TGA spectrum is consistent with that of image 3 The shape of the curve is basic...
Embodiment 3
[0070] Example 3: Preparation of linagliptin crystal form.
[0071] Add 10 g of linagliptin solid into 100 mL of n-propanol and stir at a stirring speed of 500 r / min. Raise the temperature to 50°C at a heating rate of 5°C / min to obtain a linagliptin clarified liquid, add 10 mL of purified water to the above clear liquid at a constant temperature, then cool down to 10°C at a cooling rate of 0.5°C / min, and grow crystals at a constant temperature for 2 hours. Suction filtration, and dry the obtained wet crystalline product at 35° C. and a vacuum of 0.1 Mpa for 5 hours to obtain 9.13 g of crystalline solid. The purity was 99.4%, and the yield was 91.3%.
[0072] After determination, its XRD pattern and figure 1 Basically the same, its DSC spectrum and figure 2 Basically the same, as shown by KF moisture content, its water content is about 8.0%, and its TGA spectrum is consistent with that of image 3 The shape of the curve is basically the same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com