A nickel-nitrogen codoped carbon electrocatalyst and a preparing method and application thereof
An electrocatalyst, co-doping technology, applied in chemical instruments and methods, physical/chemical process catalysts, electrodes, etc., to achieve the effect of improving selectivity, easy industrial production, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
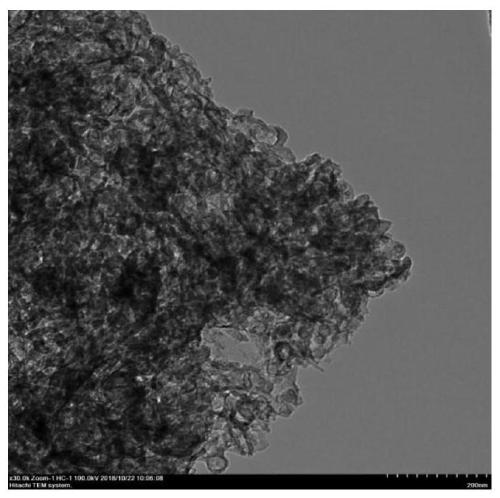
Image
Examples
Embodiment 1
[0031] The preparation steps of nickel-nitrogen co-doped porous carbon nanosheets are:
[0032] Step 1. Add 5.5mol sodium benzoate (C 7 H 5 O 2 Na), 1.56mol nickel acetate (Ni(CH 3 COO) 2 ) And 6.24mol manganese acetate (Mn(CH 3 COO) 2 ) Add 60mL N,N-dimethylformamide (DMF), the molar ratio of nickel acetate to manganese acetate is 1:4, magnetically stir until it dissolves;
[0033] Step 2. Add 11 mol of terephthalic acid (BDC) and magnetically stir to dissolve to obtain a mixed solution;
[0034] Step 3. Add the mixed solution to the hydrothermal reaction kettle and react at 180° C. for 12 hours to obtain a mixed solution for generating metal organic framework compounds;
[0035] Step 4. Centrifuge the mixed solution in step 3, wash with DMF and absolute ethanol, and then vacuum dry at 80°C for 12 hours;
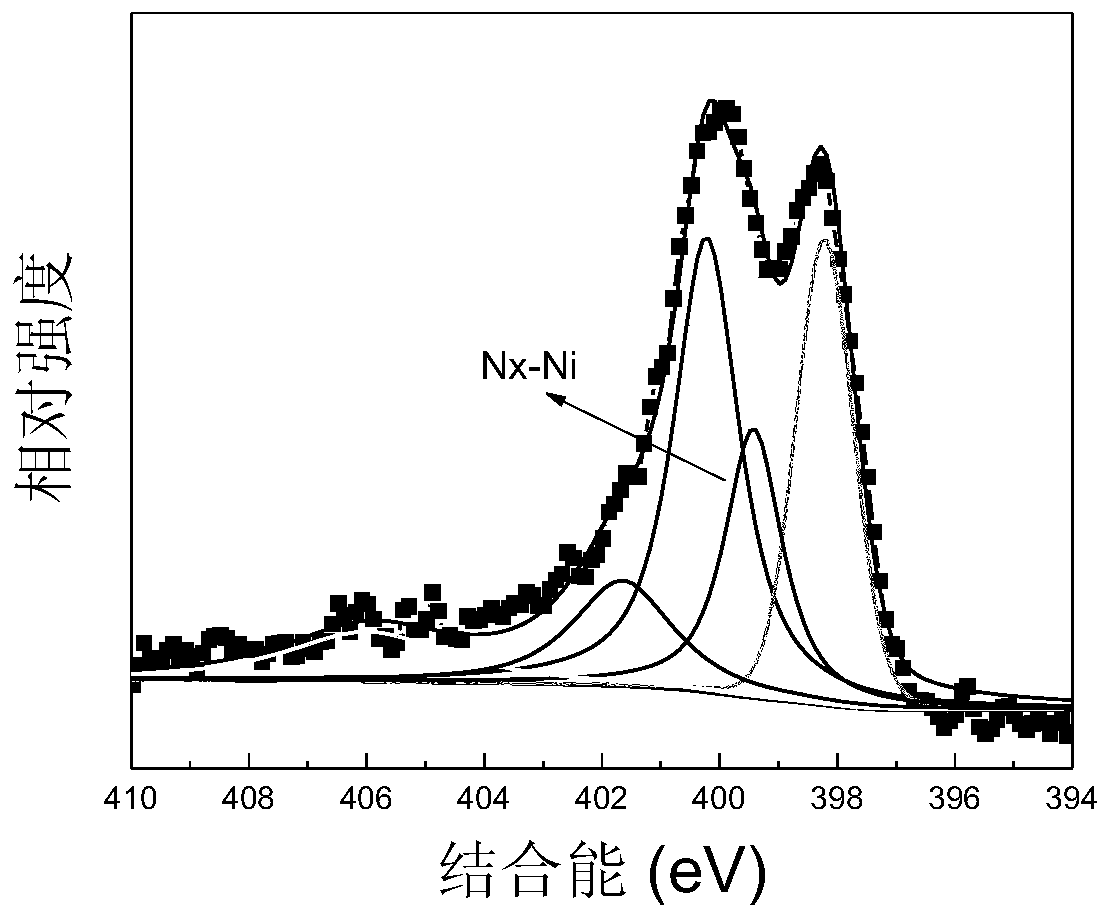
[0036] Step 5. Baking again at 700°C, the heating rate is 2.5°C / min, the baking time is 2h, and the obtained product includes a nickel-nitrogen doped carbon layer and manganese oxide...
Embodiment 2
[0046] The preparation steps of nickel-nitrogen co-doped porous carbon nanosheets are:
[0047] Step 1. Add 5.5mol sodium benzoate (C 7 H 5 O 2 Na), 0.39mol nickel acetate (Ni(CH 3 COO) 2 ) And 7.41mol manganese acetate (Mn(CH 3 COO) 2 ) Add 60mL N,N-dimethylformamide (DMF), the molar ratio of nickel acetate to manganese acetate is 1:9, magnetically stir to dissolve;
[0048] Step 2. Add 11 mol of terephthalic acid (BDC) and magnetically stir to dissolve to obtain a mixed solution;
[0049] Step 3. Add the mixed solution to the hydrothermal reaction kettle and react at 180° C. for 12 hours to obtain a mixed solution for generating metal organic framework compounds;
[0050] Step 4. Centrifuge the mixed solution in step 3, wash with DMF and absolute ethanol, and then vacuum dry at 80°C for 12 hours;
[0051] Step 5. Baking again at 700°C, the heating rate is 2.5°C / min, the baking time is 2h, and the obtained product includes a nickel-nitrogen doped carbon layer and manganese oxide;
[005...
Embodiment 3
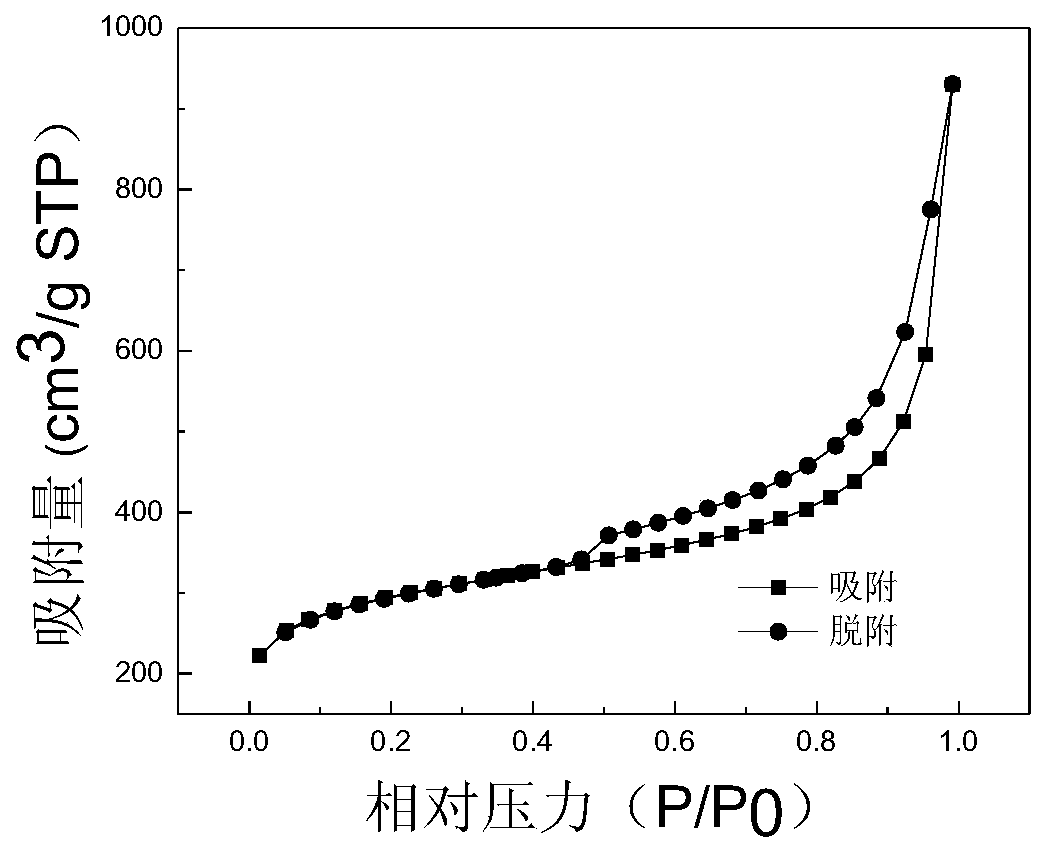
[0055] Like the nickel-nitrogen co-doped porous carbon nanosheets provided in Example 1, the molar ratio of nickel acetate to manganese acetate is 1:1.5. The heating temperature of the mixed solution B is 120°C, and the baking temperature is 600°C. The prepared nickel-nitrogen The BET specific surface area of the co-doped porous carbon nanosheets is 300m 2 / g.
[0056] As in Application Example 1, the nickel-nitrogen co-doped porous carbon nanosheets prepared in Example 3 are used as a catalyst to electrocatalyze CO reduction 2 The maximum Faraday efficiency for CO is about 85% (starting voltage is -0.5V).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com