Sulfonium salt containing pyrazoline groups and preparation method and application thereof
A technology of sulfonium salt and pyrazoline, applied in the field of new material organic chemicals, can solve the problems of difficulty in synthesis, difficulty in industrialized production, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
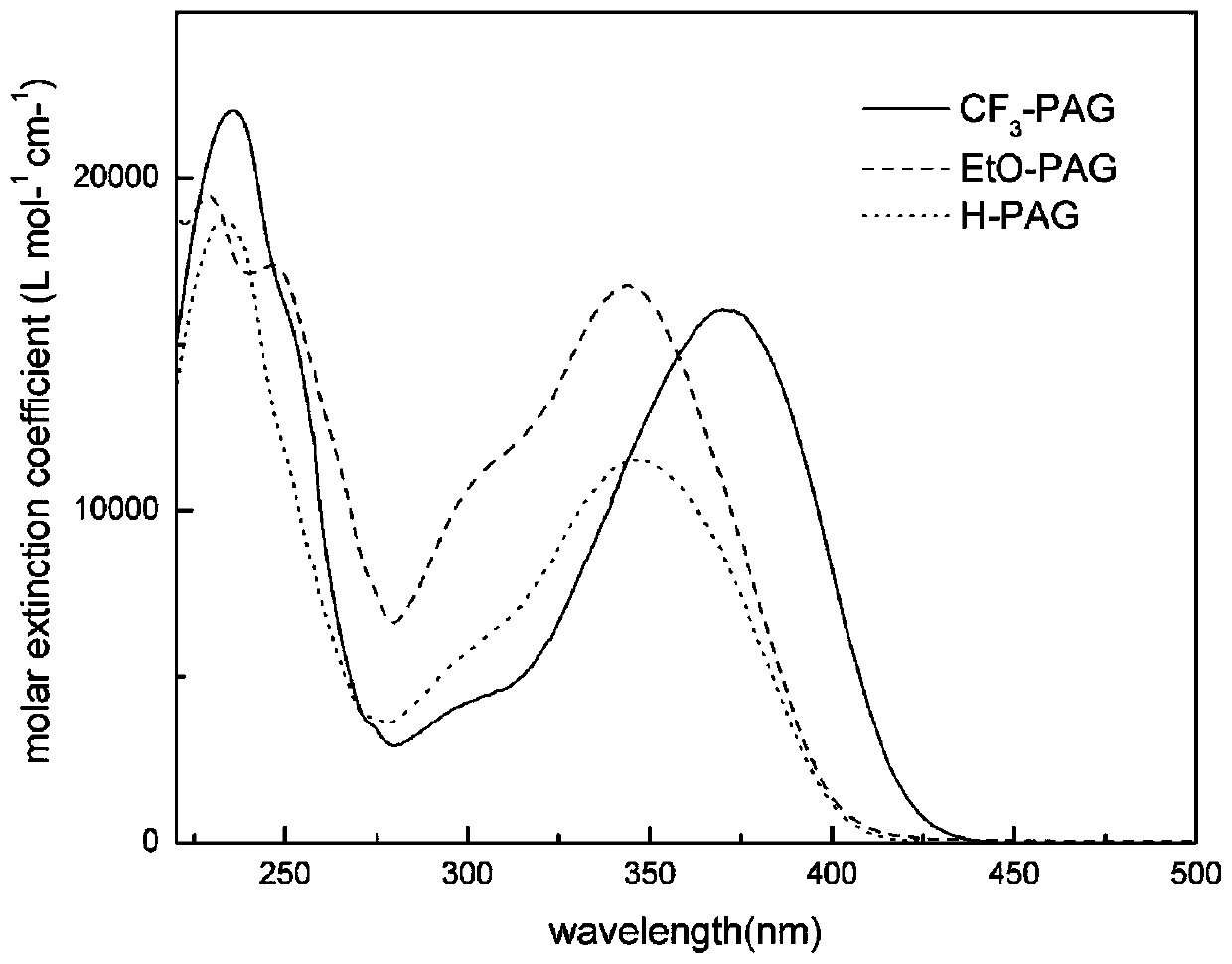
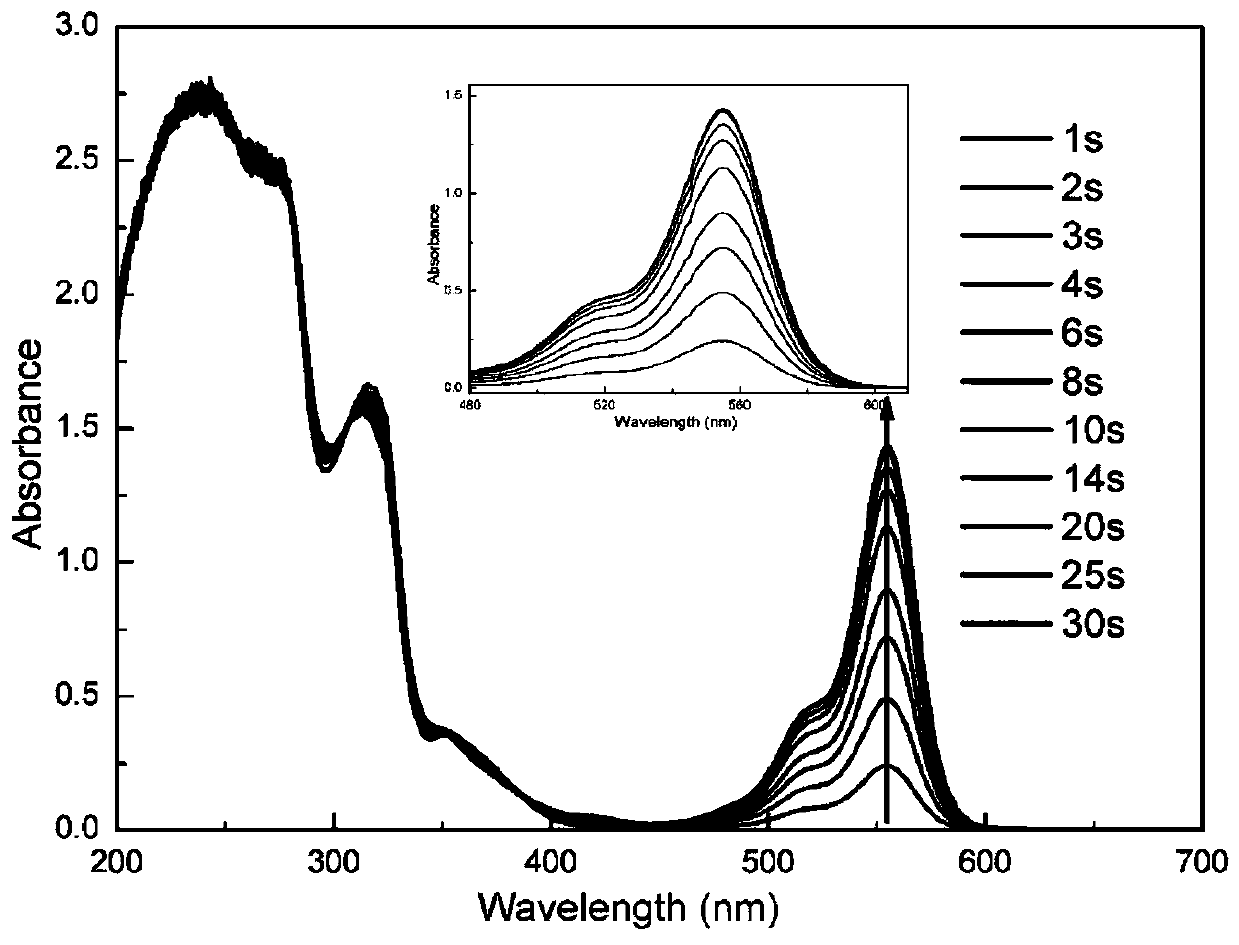
[0088] Embodiment 1: Synthesize the sulfonium salt (H-PAG) that target molecule contains pyrazoline group according to the following route:
[0089]
[0090] (a) Sodium hydroxide, absolute ethanol, normal temperature, 2h;
[0091] (b) Sodium hydroxide, absolute ethanol, 80°C, 5h;
[0092] (c) Methyl trifluoromethanesulfonate, dichloromethane, protected from light, room temperature, 24h; potassium hexafluorophosphate, room temperature.
[0093] 1. Synthesis of 3-(4-methylmercaptophenyl)-1-phenyl-2-en-1-one
[0094] Add acetophenone (12.02 g, 0.10 mol), 4-methylmercaptobenzaldehyde (15.22 g, 0.10 mol) and absolute ethanol (80 mL) into a 250 mL three-necked flask containing a magnetic rotor, and stir at room temperature. Then an aqueous solution of sodium hydroxide (8 g, 0.20 mol, 10 mL) was prepared and added dropwise to the reaction system through a constant pressure dropping funnel. After the addition was completed, the reaction was carried out for 2 hours, and the react...
Embodiment 2
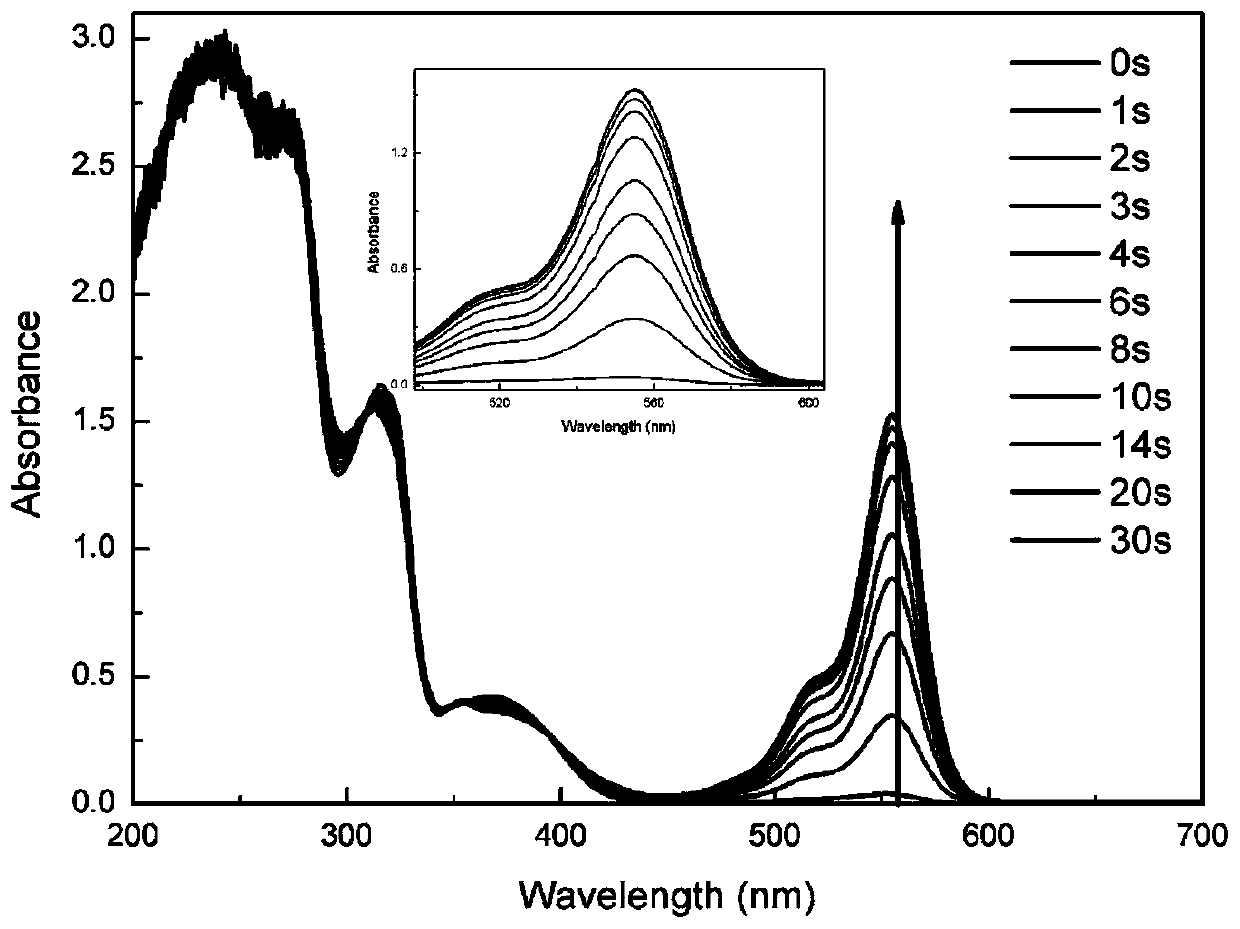
[0106] Embodiment 2: Synthesize the sulfonium salt (CF 3 -PAG):
[0107]
[0108] (a) Sodium hydroxide, absolute ethanol, normal temperature, 2h;
[0109] (b) Sodium hydroxide, absolute ethanol, 80°C, 5h;
[0110] (c) Methyl trifluoromethanesulfonate, dichloromethane, protected from light, room temperature, 24h; potassium hexafluorophosphate, room temperature.
[0111] 1. Synthesis of 3-(4-methylmercaptophenyl)-1-(4-trifluoromethylphenyl)-2-en-1-one
[0112] The product was obtained by reacting 4-trifluoromethylacetophenone and 4-methylmercaptobenzaldehyde, the method was the same as in Example 1, and the yield was 76.4%.
[0113] 1 H NMR (400MHz, Chloroform-d) δ8.08(d, J=8.1Hz, 2H), 7.78(d, J=15.7Hz, 1H), 7.75(d, J=8.1Hz, 2H), 7.55(d , J=8.5Hz, 2H), 7.43(d, J=15.7Hz, 1H), 7.25(d, J=8.1Hz, 2H), 2.51(s, 3H).
[0114] 2. Synthesis of 1-phenyl-3-(4-trifluoromethylphenyl)-5-(4-methylmercaptophenyl)-pyrazoline
[0115] Utilize 3-(4-methylmercaptophenyl)-1-(4-trifluorometh...
Embodiment 3
[0124] Embodiment 3: Synthesize the sulfonium salt (EtO-PAG) that target molecule contains pyrazoline group according to the following route:
[0125]
[0126] (a) Sodium hydroxide, absolute ethanol, normal temperature, 2h;
[0127] (b) Sodium hydroxide, absolute ethanol, 80°C, 5h;
[0128] (c) Methyl trifluoromethanesulfonate, dichloromethane, protected from light, room temperature, 24h; potassium hexafluorophosphate, room temperature.
[0129] 1. Synthesis of 3-(4-methylmercaptophenyl)-1-(4-ethoxyphenyl)-2-en-1-one
[0130] The product was obtained by reacting 4-trifluoromethylacetophenone and 4-methylmercaptobenzaldehyde, the method was the same as in Example 1, and the yield was 76.4%.
[0131] 1 H NMR (400MHz, Chloroform-d) δ8.05(d, J=9.9Hz, 2H), 7.78(d, J=15.6Hz, 1H), 7.58(d, J=8.9Hz, 2H), 7.53(d ,J=15.6Hz,1H),7.27(d,J=8.9Hz,2H),6.99(d,J=9.9Hz,2H),4.14(q,J=7.0Hz,2H),2.54(s,3H ), 1.47 (t, J=7.0Hz, 3H).
[0132] 2. Synthesis of 1-phenyl-3-(4-ethoxyphenyl)-5-(4-met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com