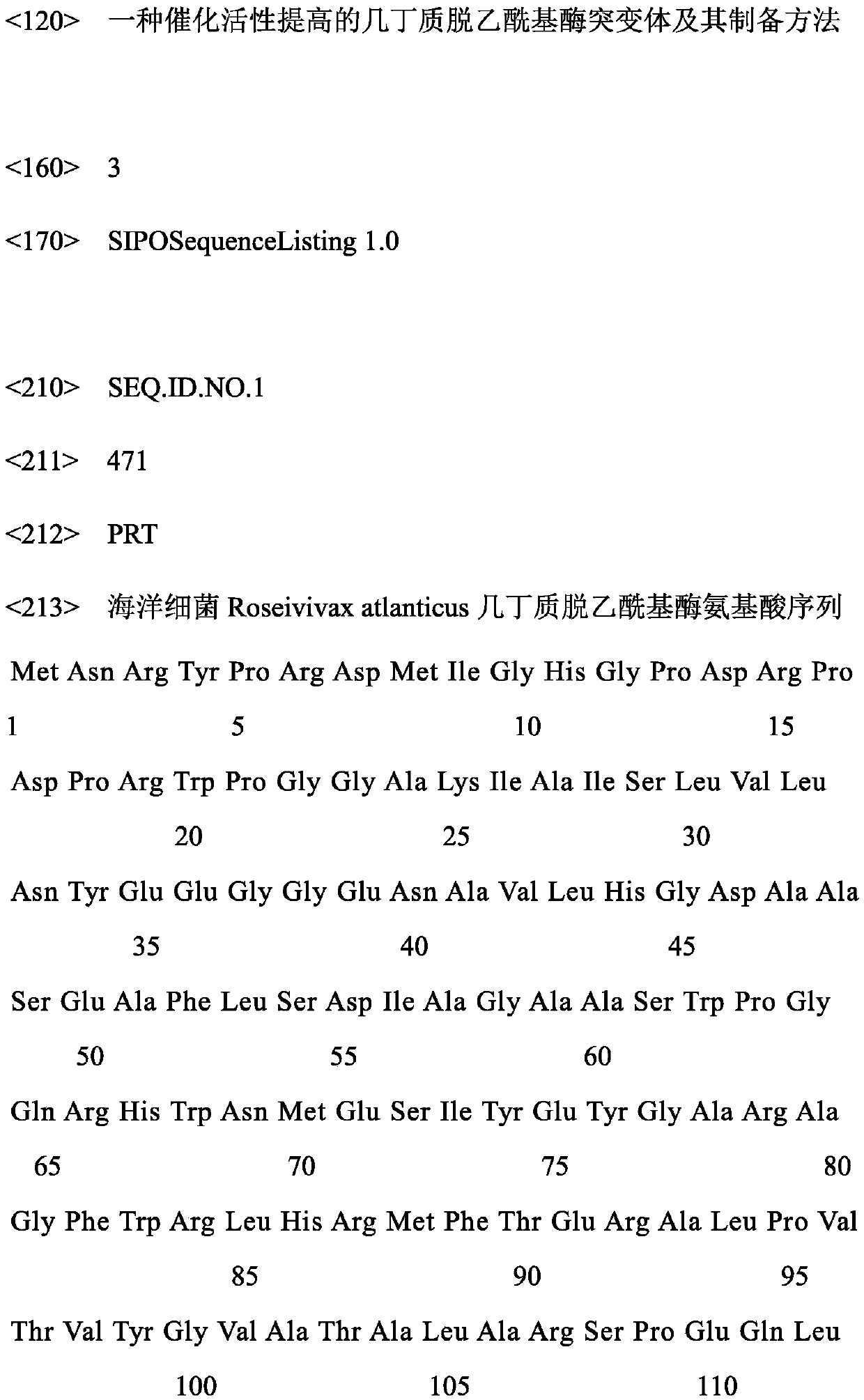
Chitin deacetylase mutant with improved catalytic activity and preparation method thereof
A technology for improving catalytic activity and deacetylase, which is applied in the field of bioengineering to achieve the effects of strong penetrating power, favorable application and improved catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The construction of embodiment 1 mutant expression plasmid and the acquisition of recombinant Escherichia coli
[0028]Primers were designed according to the wild-type enzyme gene, and the restriction site was introduced. The primers used were as follows: P1: 5'-CGGAATTCATGAACCGTTACCCGCGTGAC-3' (introduced restriction site EcoR I); P2: 5'-CCCATTGTTAAGCCAGAGCTTCCAGACGC-3' (introduced Noc I restriction site).
[0029] Use the synthesized DNA sequence as a template for PCR, and the PCR reaction system is: ddH 2 O (17.5μL), buffer (2.5μL), Mg 2+ (2.5 μL), dNTP (0.5 μL), P1 (0.5 μL), P2 (0.5 μL), template (1 μL), Taq enzyme (0.2 μL). PCR program: pre-denaturation at 94°C for 5 minutes, followed by 35 cycles at 94°C for 30s, 54°C for 40s, and 72°C for 70s; extension at 72°C for 10 minutes. The amplified products were detected by 1.0% agarose gel electrophoresis and then sequenced.
[0030] The above PCR amplified product was subjected to 1.0% agarose gel electrophoresis, ...
Embodiment 2
[0048] Transformation of embodiment 2 mutant expression vector
[0049] The medium composition used: tryptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH7.0.
[0050] The recombinant bacterium E.coli BL21(DE3)(pEtac-His6-cad) containing the gene encoding the mutant chitin deacetylase was activated and cultured and then inoculated into the fermentation medium containing 100 μg mE-1 ampicillin, and filled with liquid The amount is 30mL / 250mL, cultured at 37°C, 200r min-1; the bacteria grow to OD600=0.6, add final concentration of 0.5mM IPTG, 30°C, induce fermentation for 24h. 180r / min induced expression for 5h. Cells were collected by refrigerated centrifugation at 10,000 r / min at 4°C for 5 min.
[0051] Resuspend the bacteria in distilled water, crush the bacteria with an ultrasonic cell disruptor (200W, super 3s, stop 7s, ultrasonic 5min in total), centrifuge at 10000r / min at 4°C for 20min, and collect the supernatant as a crude enzyme solution.
[0052] According to Ni-NTA P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com