Method for synthesizing ferric phosphate by utilizing waste phosphate generated by precipitation of trace heavy metal
A waste phosphate and heavy metal technology, which is applied in the direction of phosphorus compounds, non-metallic elements, chemical instruments and methods, etc., can solve the problems of high production cost and small particle size of iron phosphate, and achieve qualified product quality, simple process and good industrial The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
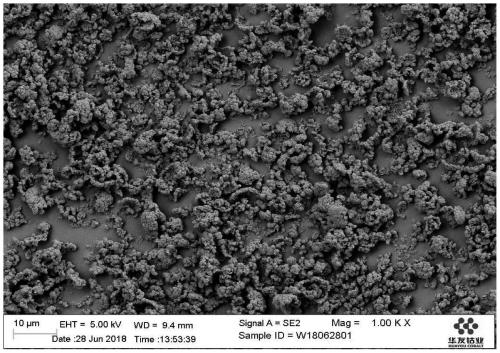
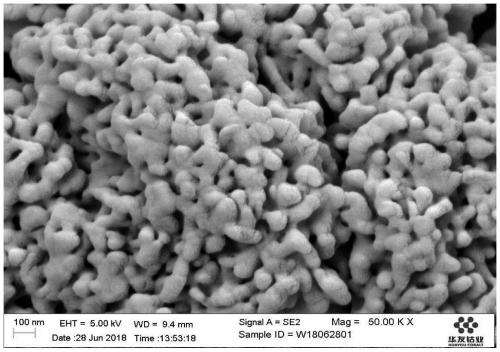
Image
Examples
Embodiment 1
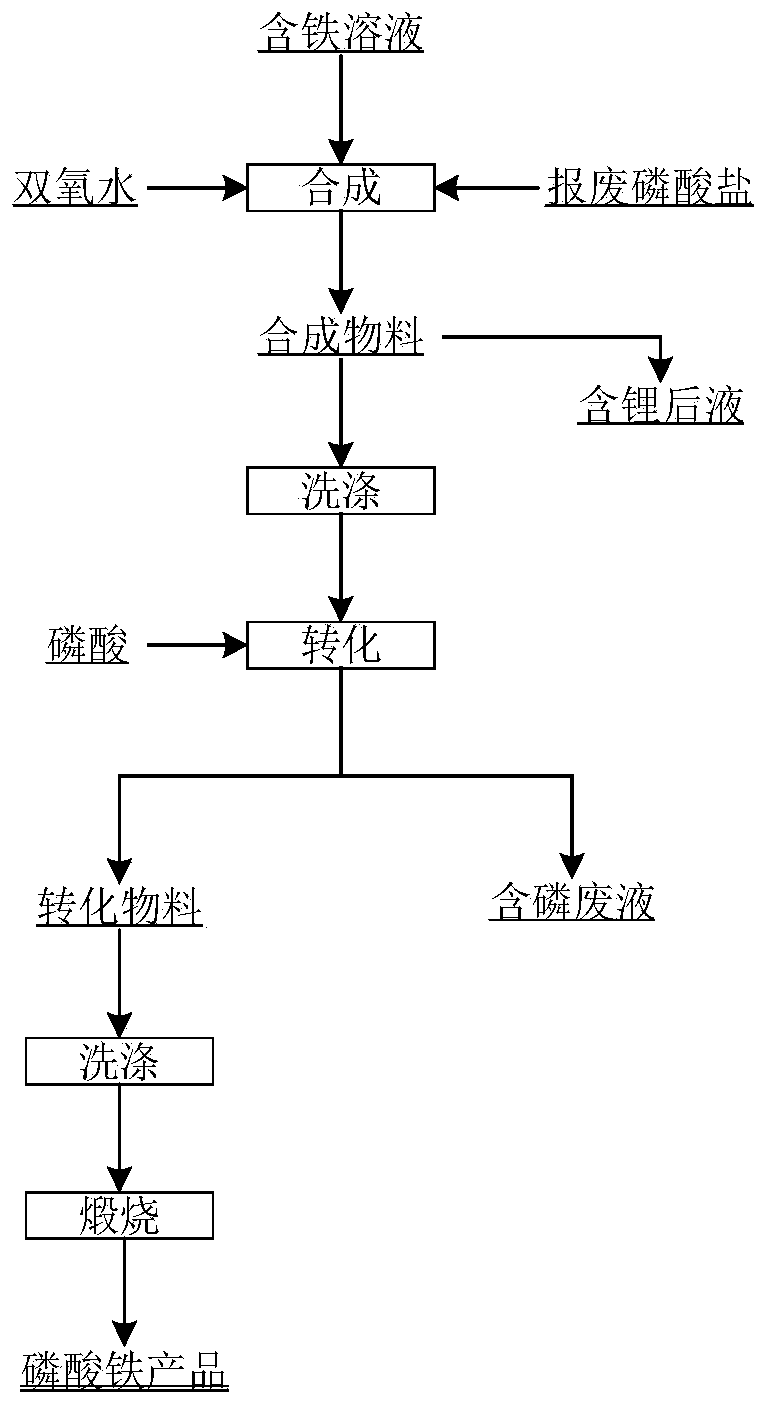
[0031] In this embodiment, the iron source is ferrous sulfate solution, and the phosphorus source is scrap lithium phosphate slag.
[0032] 1) Prepare 75g / L ferrous sulfate solution, wash the scrap lithium phosphate residue with water 1-2 times at the same time, add sulfuric acid to the filter residue after filtration to dissolve it.
[0033] 2) Add tap water + a small amount of hydrogen peroxide to make a base in the reactor. During the stirring process, add waste phosphate solution, 2.5mol ferrous sulfate solution and hydrogen peroxide into the reactor for synthesis. 1. The amount of hydrogen peroxide used is 1.5-2.0 times the amount of ferrous substances in the iron-containing solution, the feeding temperature is 40°C-50°C, the feeding time is 10min, the stirring speed is 240rpm, and the pH value of the process is 2.3-2.6. After the synthesis, the material is reacting After the kettle was kept warm for 60 minutes, the synthetic material and lithium-containing liquid were ob...
Embodiment 2
[0041] The iron source used in the present embodiment is a white metal leaching solution, and the phosphorus source is scrap lithium phosphate slag, and the main components of the white metal leaching solution are shown in Table 2:
[0042] Table 2 Contents of main elements in white alloy leaching solution (g / L)
[0043] element
co
Cu
Ni
Fe
Al
mn
Ca
Mg
Zn
content
37.71
0.0067
0.016
112.3
0.55
0.35
0.72
0.76
0.65
[0044] 1) Adjust the pH of the white metal leaching solution to 4-5 with cobalt carbonate at room temperature, then add iron powder and stir for 0.5 hours at room temperature, then filter with suction. After the filter, adjust the pH of the filtrate to 2.8 to obtain purified liquid , diluted to 72g / L.
[0045] 2) Wash the scrap lithium phosphate slag with water for 1-2 times, and add sulfuric acid to the filter residue after filtering to dissolve it.
[0046]3) Add tap water + a smal...
Embodiment 3
[0054] In this embodiment, the iron source is ferric chloride solution, and the phosphorus source is scrap lithium phosphate slag.
[0055] 1) Prepare a 75g / L ferric chloride solution, and at the same time wash the scrap lithium phosphate residue with water for 1 to 2 times, and add phosphoric acid to the filter residue after filtration to dissolve it.
[0056] 2) Add tap water into the reaction kettle, and feed the waste phosphate solution and 2.5mol ferric chloride solution into the reaction kettle to synthesize during the stirring process. During the process, keep the molar ratio of iron and phosphorus as 1, and the feeding temperature is 40°C-50°C , the feeding time is 10min, the stirring speed is 240rpm, and the pH value of the process is 2.3-2.6. After the synthesized material is kept warm in the reactor for 60min, the synthesized material and lithium-containing liquid are obtained by suction filtration.
[0057] 3) Wash the synthesized material at a temperature of 40-70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com