A kind of enantiomeric mixture and its preparation method and application
A mixture and enantiomer technology, applied in the field of enantiomer mixture and its preparation, can solve the problems of low drug efficacy and high application amount, achieve the effects of small environmental impact, mild reaction conditions, and reduce pesticide application amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
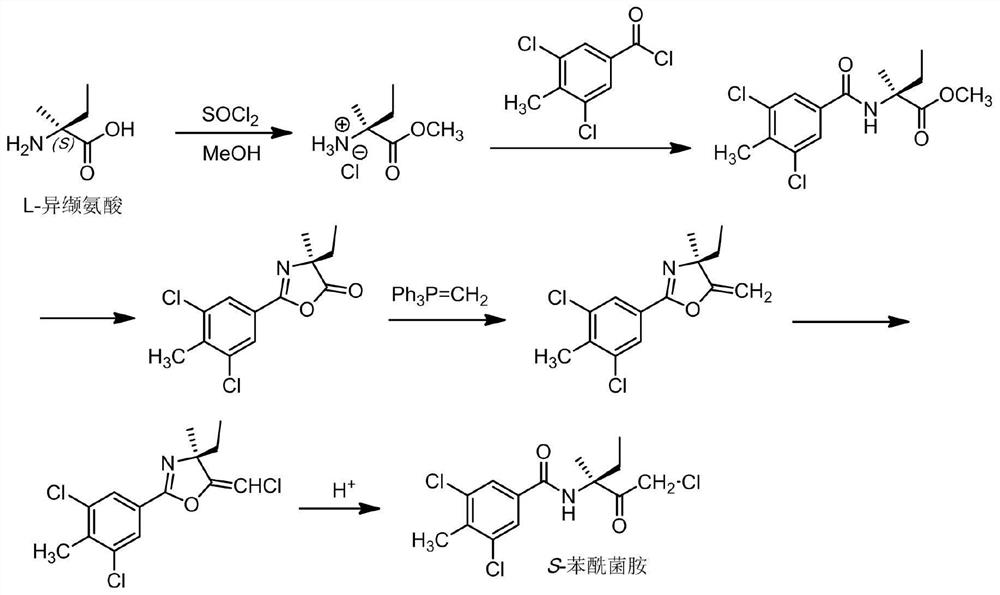
[0039] The present invention also provides a method for preparing the enantiomeric mixture described in the above technical scheme, comprising the following steps:
[0040] Esterifying isovaline with methanol in the presence of thionyl chloride to obtain isovaline methyl ester hydrochloride, the isovaline being L-isovaline or D-isovaline ;
[0041] The isovaline methyl ester hydrochloride is subjected to amidation reaction with 3,5-dichloro-4-methylbenzoyl chloride in a buffer solution to obtain 2-(3,5-dichloro-4-methyl Methylbenzoyl)-2-methylbutyrate;
[0042] The 2-(3,5-dichloro-4-methylbenzoyl)-2-methylbutanoic acid methyl ester is subjected to a cyclization reaction to obtain 2-(3,5-dichloro-4-methyl Phenyl)-4-ethyl-4-methyloxazol-5(4H)-one;
[0043] The 2-(3,5-dichloro-4-methylphenyl)-4-ethyl-4-methyloxazol-5(4H)-one and methyl Wittig reagent were carried out under alkaline conditions Wittig reaction to obtain 2-(3,5-dichloro-4-methylphenyl)-4-ethyl-4-methyl-5-methyle...
Embodiment 1
[0085] Embodiment 1: the preparation of S-benzamid
[0086] (1) Esterification reaction:
[0087] Suspend 117 g (1 mol) of L-isovaline in 1,500 mL of methanol, and slowly add 179 g (1.5 mol) of thionyl chloride to the suspension with sufficient stirring. After the addition, the temperature was raised to reflux for 3h. The resulting reaction mixture was cooled to room temperature and filtered. The filter cake was washed several times with methanol. The filtrate and methanol washings were combined and concentrated under reduced pressure. Toluene was added to the obtained residue, followed by stirring. Toluene was removed to obtain 159 g of the expected product L-isovaline methyl ester hydrochloride (yield 95%).
[0088] (2) Amidation reaction:
[0089] A. Inorganic acid-binding agent method:
[0090] Add an aqueous solution of sodium bicarbonate prepared from 13.25 g of sodium bicarbonate and 100 mL of water, and 200 mL of solvent methyl isobutyl ketone into the reactor. ...
Embodiment 2
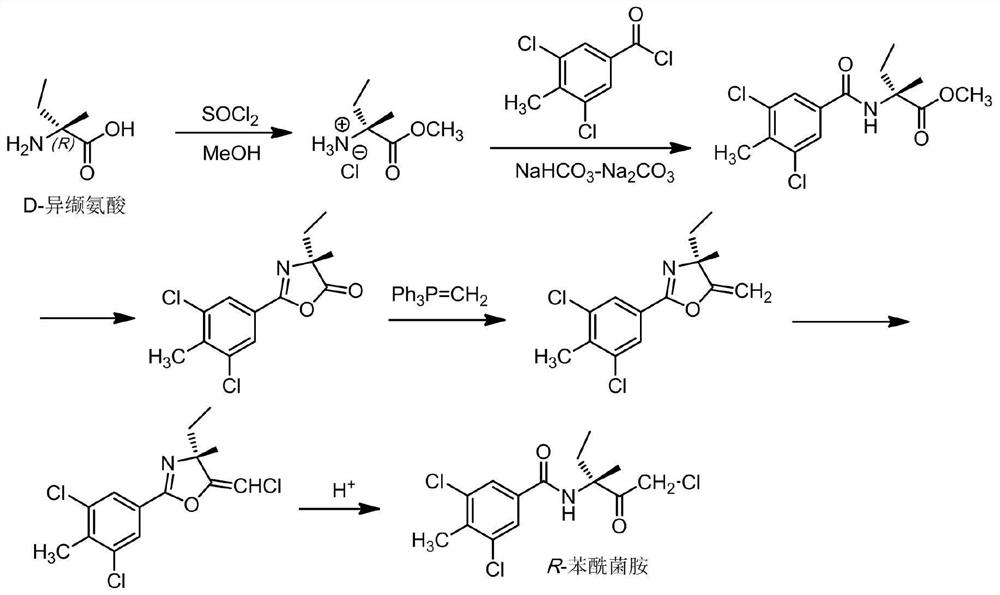
[0104] Embodiment 2: the preparation of R-benzamid
[0105] (1) Esterification reaction:
[0106] 117 g (1 mol) of D-isovaline was suspended in 1500 mL of methanol. Under thorough stirring, 179 g (1.5 mol) of thionyl chloride was slowly added to the suspension. After the addition, the temperature was raised to reflux for 3h. The resulting reaction mixture was cooled to room temperature and filtered. The filter cake was washed several times with methanol. The filtrate and methanol washings were combined and concentrated under reduced pressure. Toluene was added to the obtained residue, followed by stirring. The toluene was removed to obtain 156 g of the expected product D-isovaline methyl ester hydrochloride (yield 93%).
[0107] (2) Amidation reaction:
[0108] With L-isovaline methyl ester hydrochloride 140g (0.83mol) and 3,5-dichloro-4-methylbenzoyl chloride 190.5g (0.853mol) and methyl isobutyl ketone that were made in the previous step Add each 1.0 L dropwise to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com