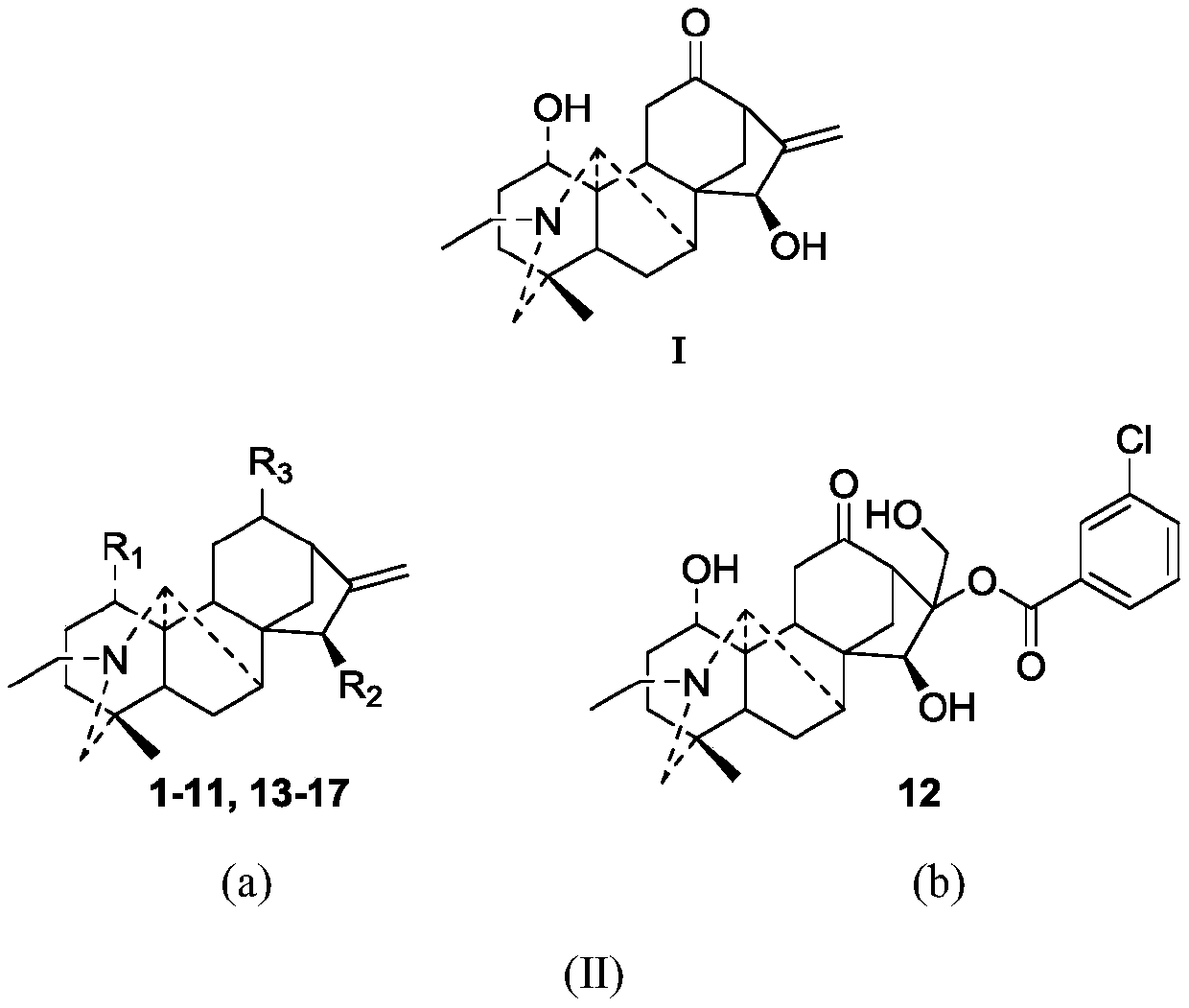
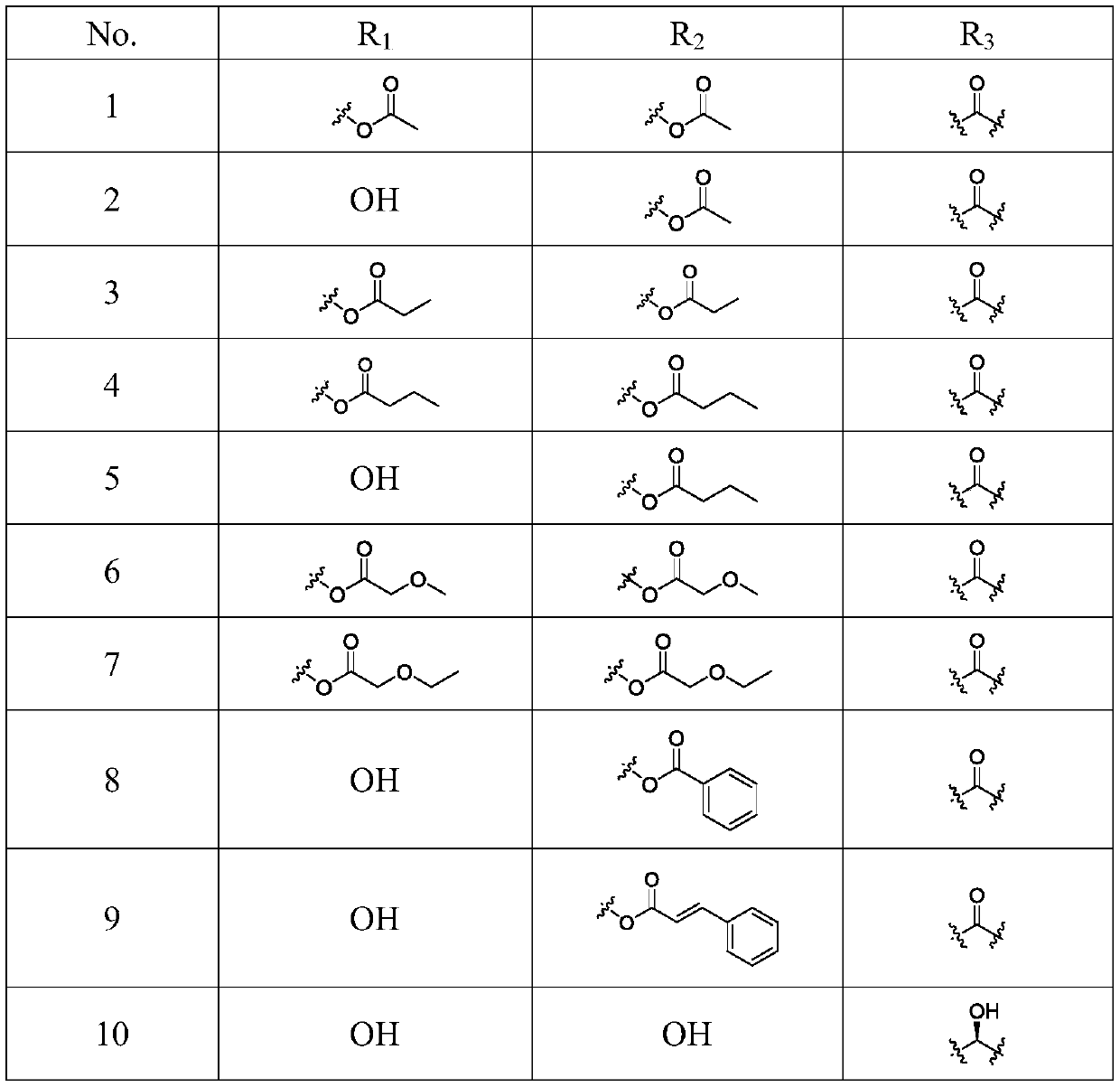
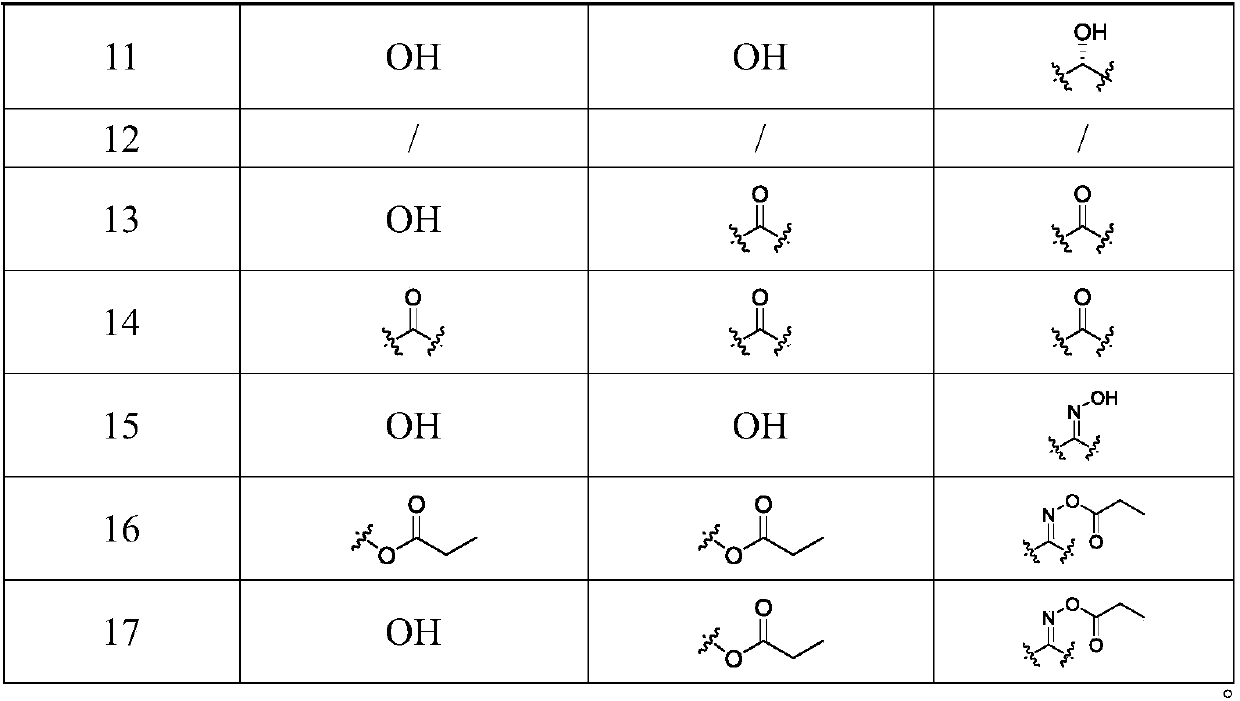
Songorine derivatives, and pharmaceutical composition and applications thereof
A derivative, the technology of Songguolin, applied in the field of Songguolin derivatives and pharmaceutical compositions thereof, can solve the problems of lack of type II diabetes drugs, no Songguolin derivatives, and no reports of B-type G protein-coupled receptor inhibitors, etc. Achieve high clinical efficiency and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] Molecular formula: C 26 h 35 NO 5
[0049] Molecular weight: 441
[0050] Appearance: white amorphous powder
[0051]
[0052] Molecular formula: C 24 h 33 NO 4
[0053] Molecular weight: 399
[0054] Appearance: white amorphous powder
[0055] resolve resolution:
[0056] Compound I (50 mg, 0.14 mmol) and 4-dimethylaminopyridine (DMAP, 17.1 mg, 0.14 mmol) were dissolved in anhydrous pyridine (2 mL), and acetyl chloride (0.2 mL, 0.34 mmol) was slowly added dropwise at room temperature, The reaction solution was stirred at room temperature until the reaction of the raw materials was complete. The reaction solution was slowly poured into ice water (20mL), extracted with dichloromethane (20mL×3), the dichloromethane layer was washed with saturated sodium bicarbonate (20mL×3) and saturated brine (15mL) successively, and washed with anhydrous After drying over sodium sulfate and concentrating under reduced pressure, it was subjected to silica gel c...
Embodiment 2
[0061]
[0062] Molecular formula: C 28 h 39 NO 5
[0063] Molecular weight: 469
[0064] Appearance: white amorphous powder
[0065] resolve resolution:
[0066] Compound I (50mg, 0.14mmol) and DMAP (17.1mg, 0.14mmol) were dissolved in anhydrous pyridine (2mL), propionic anhydride (0.29mL, 0.34mmol) was slowly added dropwise at room temperature, and the reaction solution was stirred at room temperature to react until the raw materials are completely reacted. The reaction solution was slowly poured into ice water (20mL), extracted with dichloromethane (20mL×3), the dichloromethane layer was washed with saturated sodium bicarbonate (20mL×3) and saturated brine (15mL) successively, and washed with anhydrous After drying over sodium sulfate and concentrating under reduced pressure, silica gel column chromatography with eluent petroleum ether / ethyl acetate / diethylamine (15:1:1) gave 310.5 mg of a white solid compound with a yield of 16%.
[0067] Spectral data:
[0068]...
Embodiment 3
[0070]
[0071] Molecular formula: C 30 h 43 NO 5
[0072] Molecular weight: 497
[0073] Appearance: white amorphous powder
[0074]
[0075] Molecular formula: C 26 h 37 NO 4
[0076] Molecular weight: 427
[0077] Appearance: white amorphous powder
[0078] resolve resolution:
[0079]Compound I (50mg, 0.14mmol) and DMAP (17.1mg, 0.14mmol) were dissolved in anhydrous pyridine (2mL), and butyric anhydride (0.55mL, 0.34mmol) was slowly added dropwise at room temperature, and the reaction solution was stirred at room temperature to react until the raw materials are completely reacted. The reaction solution was slowly poured into ice water (20mL), extracted with dichloromethane (20mL×3), the dichloromethane layer was washed with saturated sodium bicarbonate (20mL×3) and saturated brine (15mL) successively, and washed with anhydrous After drying over sodium sulfate and concentrating under reduced pressure, it was subjected to silica gel column chromatography, e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com