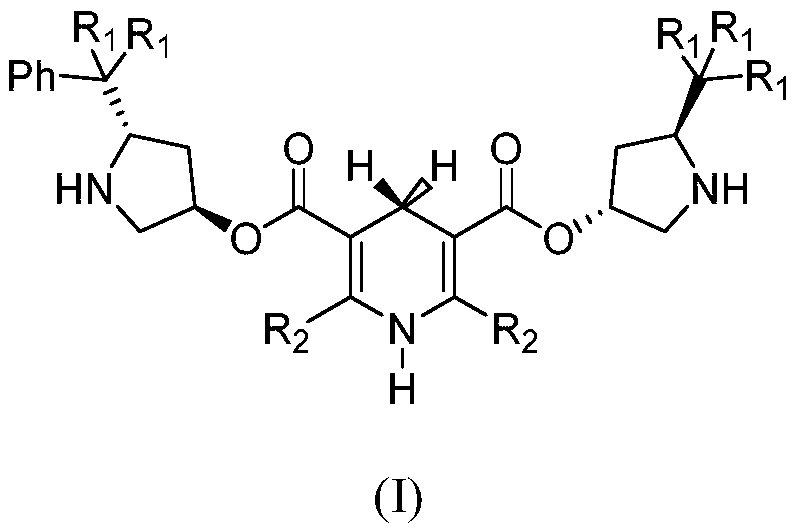
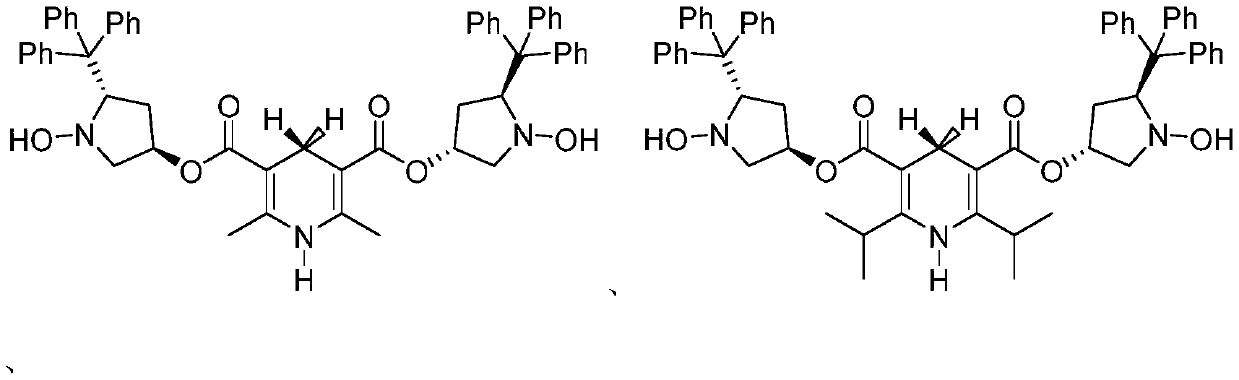
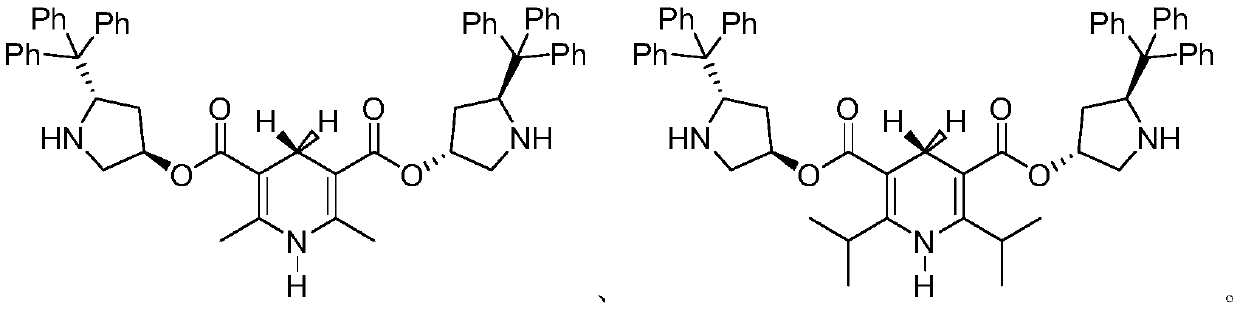
1, 4-dihydropyridine bifunctional chiral catalyst and preparation method and application thereof
A dihydropyridine and catalyst technology, which is applied in the directions of organic compound/hydride/coordination complex catalyst, carbon-based compound preparation, chemical instruments and methods, etc. Recycling, difficult to control hybrid effects, etc., to avoid transition metal residues, easy to regenerate and reuse, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Molecular structural formula of (3R,5S)-5-trityl-1,3-dihydroxypyrrolidine:
[0052]
[0053] 2. Preparation: Slowly drop 1M tetrabutylammonium fluoride solution in tetrahydrofuran (4mL, 4mmol) into (2S,4R)-4-dimethyltert-butylsilyloxy-2-triphenyl-1-hydroxy A solution of pyrrolidine (459.7 mg, 1 mmol) in dry THF (10 mL). The reaction was vigorously stirred at room temperature for 10 hours, concentrated under reduced pressure, and the crude product was purified by silica gel column chromatography (the volume ratio of n-hexane:ethyl acetate was 20:1) to obtain white (3R,5S)-5-trityl Base-1,3-dihydroxypyrrolidine solid 338mg (III), yield 98%.
[0054] 3. Structural identification: the obtained compound structure was verified by nuclear magnetic resonance ( 1 (H-NMR) characterization result is: 1 H NMR (400MHz, CDCl 3 )δ7.39(t, J=8.4Hz, 6H), 7.31(t, J=8.4Hz, 3H), 7.19(d, J=8.4Hz, 6H), 6.88(brs, 1H), 5.26(brs, 1H), 4.37(t, J=9.6Hz, 1H), 3.95-3.93(m, 1H), 2.69(dd, J...
Embodiment 2
[0056] 1. The molecular structural formula of (3R,5S)-1-hydroxy-3-acetoacetoxy-5-triphenylmethylpyrrolidine:
[0057]
[0058] 2. Preparation: Add 2,2,6-trimethyl-1,3-dioxin-4-one (142.2 mg, 1 mmol) dropwise to (3R,5S)-5-trityl-1, In a solution of 3-dihydroxypyrrolidine (345.4 mg, 1 mmol) in xylene (0.5 mL) at 130°C. The acetone formed was distilled off from the reaction mixture. Stirring was continued at 130°C for 2 hours, the reaction mixture was cooled to 50°C and the solvent was removed in vacuo. The crude product was purified by silica gel column chromatography (the volume ratio of n-hexane:ethyl acetate was 20:1) to obtain 365 mg of light yellow solid compound (IV), with a yield of 85%.
[0059] 3. Structural identification: the obtained compound structure was verified by nuclear magnetic resonance ( 1 (H-NMR) characterization result is: 1 H NMR (400MHz, CDCl 3 )δ7.43(t, J=8.4Hz, 6H), 7.32(t, J=8.4Hz, 3H), 7.18(d, J=8.4Hz, 6H), 6.89(brs, 1H), 4.37(t, J=9.6Hz, 1H...
Embodiment 3
[0061] 1. Molecular structural formula of (3R,5S)-1-hydroxy-3-isobutyrylacetoxy-5-triphenylmethylpyrrolidine:
[0062]
[0063] 2. Preparation: Add 2,2-dimethyl-6-isopropyl-1,3-dioxin-4-one (170.2mg, 1mmol) dropwise to (3R,5S)-5-trityl In a solution of 1,3-dihydroxypyrrolidine (345.4mg, 1mmol) in xylene (0.5mL) at 130°C. Stirring was continued at 130°C for 2 hours, the reaction mixture was cooled to 50°C and the solvent was removed in vacuo. The crude product was purified by silica gel column chromatography (the volume ratio of n-hexane:ethyl acetate was 20:1) to obtain 297 mg of light yellow solid compound (IV), with a yield of 65%.
[0064] 3. Structural identification: the obtained compound structure was verified by nuclear magnetic resonance ( 1 (H-NMR) characterization result is: 1 H NMR (400MHz, CDCl3) δ7.40(t, J=8.4Hz, 6H), 7.31(t, J=8.4Hz, 3H), 7.16(d, J=8.4Hz, 6H), 6.90(brs, 1H ), 4.36(t, J=9.6Hz, 1H), 3.95-3.92(m, 1H), 3.46(s, 2H), 2.71(dd, J=11.6, 6.0Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com