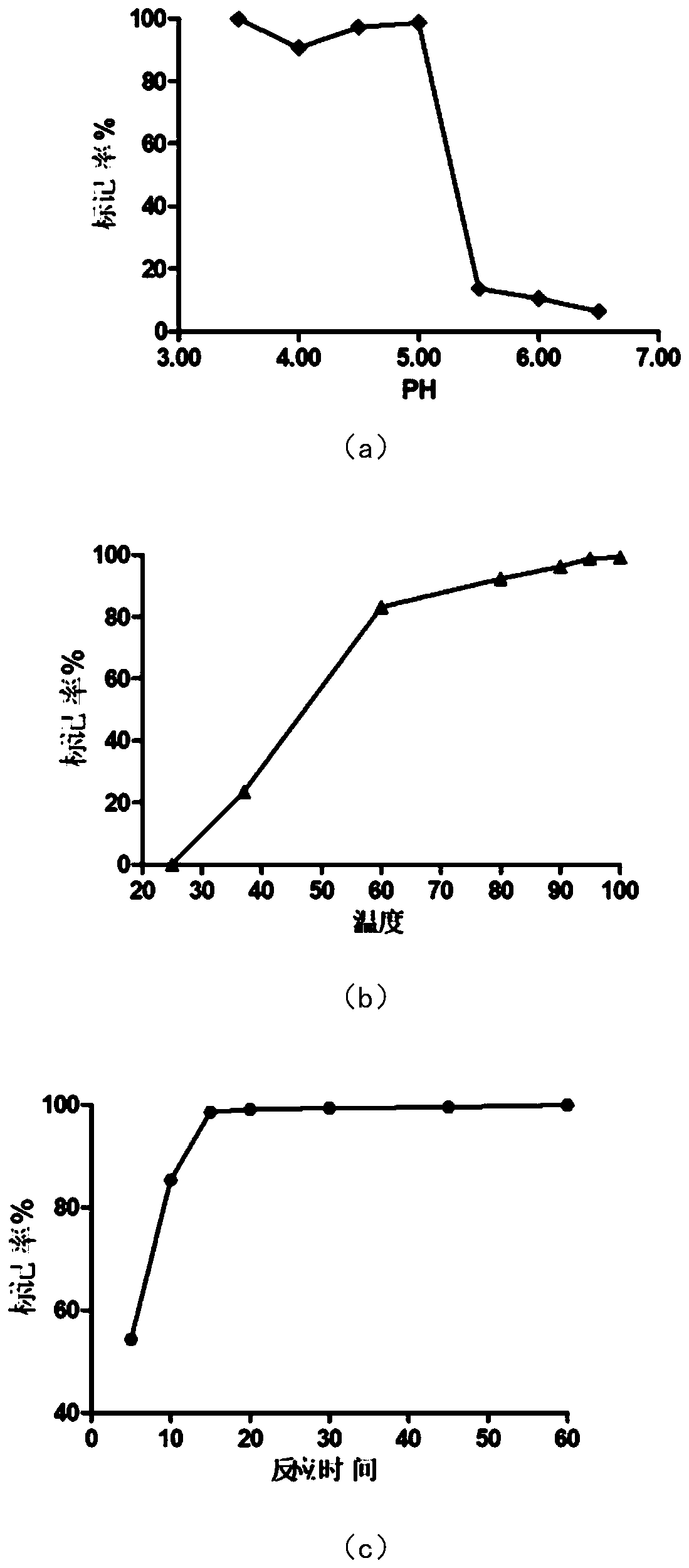
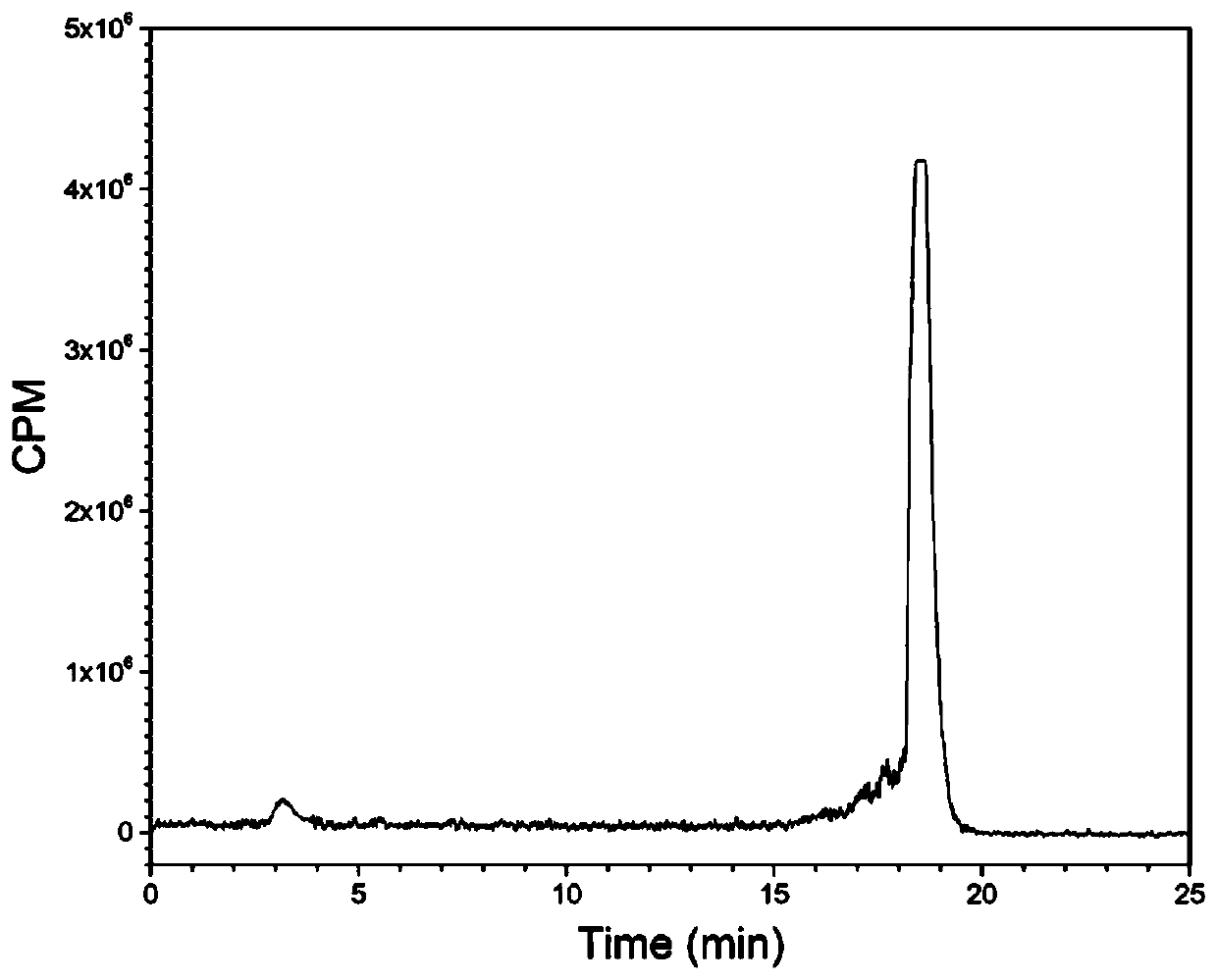
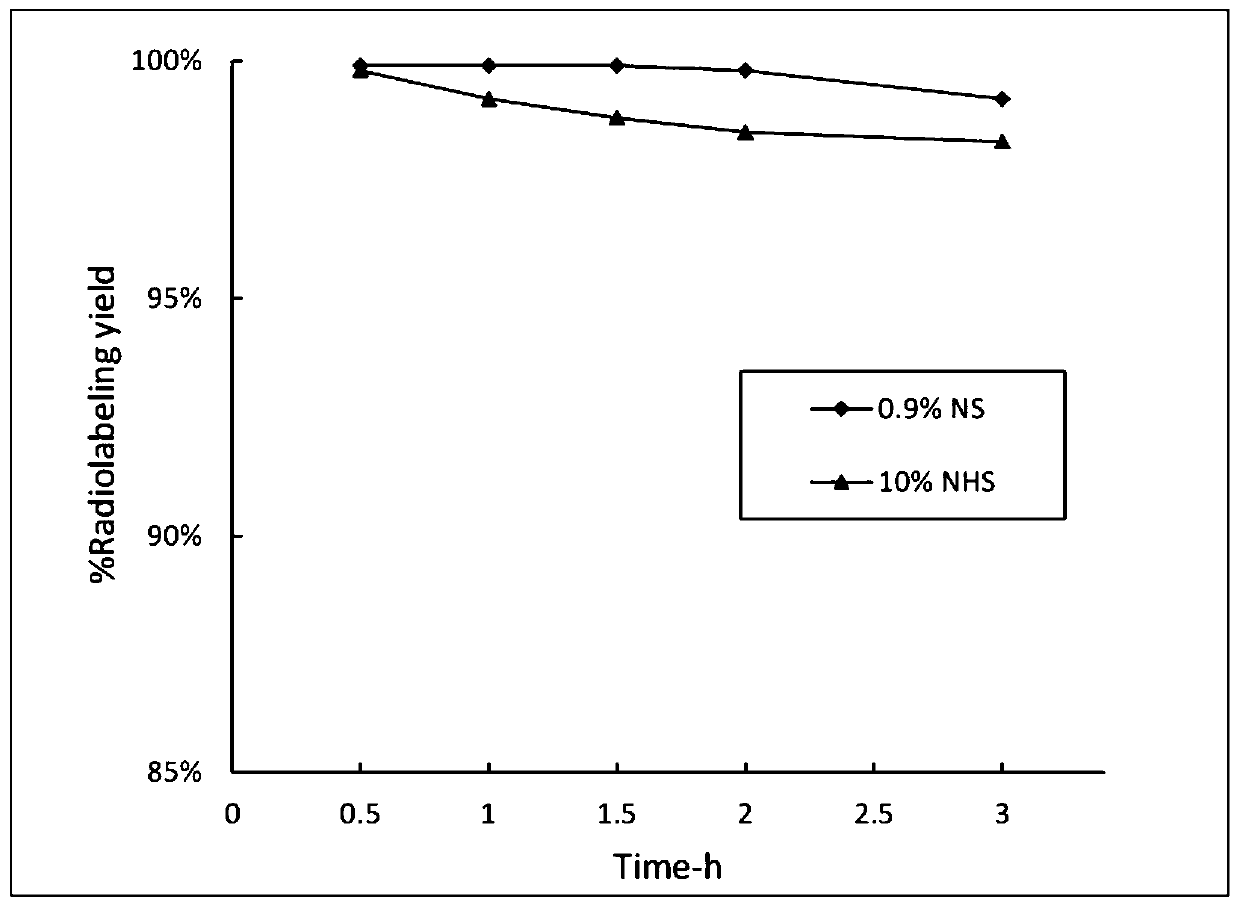
Somatostatin precursor compound and somatostatin ligand compound of octreotide and preparation and application thereof
A technology of ligand compounds and precursor compounds, which is applied in the field of radiopharmaceuticals and nuclear medicine, can solve the problems of unfavorable reaction temperature for biomolecules, more Ga decay during labeling time, general thermodynamic stability, etc., and achieve easy automatic synthesis and stable labeling products , Conducive to the effect of commercial application and clinical promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 168
[0044] Example 1 68 Preparation of Ga-NOTA-TATE
[0045] 1. Synthesis of NOTA-TATE
[0046] NOTA-TATE was synthesized by standard FMOC solid-phase peptide synthesis method, the specific steps are as follows:
[0047] 1) Dissolve Throl-resin and Fmoc-amino acid in N,N-dimethylformamide, add HATU, and condense at room temperature for 4 hours.
[0048] 2) Wash the condensation reaction product 3 times with N,N-dimethylformamide, add N,N-dimethylformamide (DMF) / piperidine / piperidine at a volume ratio of 96:2:2 The mixed solution of diazabicyclo (DBU) was deprotected, and after incubating in a 37° C. incubator for 5 minutes, the solvent was removed, and the coupling steps were repeated sequentially according to the desired peptide sequence.
[0049] 3) Add a mixed solution of dichloromethane / acetic acid / trifluoroethanol with a volume ratio of 3:1:1, stir gently for 2 hours, cleave the polypeptide from the resin, and incubate overnight at room temperature with dimethyl sulfoxide ...
Embodiment 268
[0061] Example 2 68 Micro-PET / CT imaging of Ga-NOTA-TATE in normal mice
[0062] To the tail vein of normal adult mice, inject the compound prepared in Example 1 68 Ga-NOTA-TATE 100uCi / mouse, observe the distribution of ligand compounds in normal mice at different time points after injection.
[0063] attached Figure 4 for injection 68 Micro-PET / CT images of normal mice 10min, 30min, 1h, 2h after Ga-NOTA-TATE. From attached Figure 4 It can be seen that in 68Imaging of mice 10 minutes after Ga-NOTA-TATE injection showed increased radioactive distribution in the heart blood pool, liver, and kidneys, increased radioactive discharge into the bladder, mildly increased radioactive distribution in the brain, lungs, small intestine, and muscles, and extremities and bones There was no increase in the distribution of radioactivity in the spine and spine; at 30 minutes of injection, the distribution of radioactivity in the blood pool of the liver and heart decreased, and the dist...
Embodiment 368
[0064] Example 3 68 Distribution of Ga-NOTA-TATE in normal adult rats
[0065] Six normal adult rats, half male and half male, were pre-anesthetized with 3% isoflurane-oxygen mixed gas, placed on the PET / CT scanning bed, and treated with 1.5% isoflurane Anesthesia was maintained with isoflurane-oxygen mixture and respiration was monitored. Inject 400uCi / cat by tail vein 68 Immediately after Ga-NOTA-TATE, Micro-PET / CT dynamic imaging was performed for 4 hours. After acquisition and image reconstruction, use the software provided by the supplier to delineate the myocardium, blood pool, liver, kidney, brain, muscle, bone, stomach, and small intestine on the whole-body attenuation-corrected coronal image as regions of interest (ROI). Get it 68 Ga-NOTA-TATE in 30s, 5min, 10min, 30min, 1h, 2h, 4h per gram of tissue injection dose percentage (%ID / g), the results are shown in Table 1 and Figure 5 shown.
[0066] Table 1 68 Biodistribution of Ga-NOTA-TATE in normal rats viscer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com