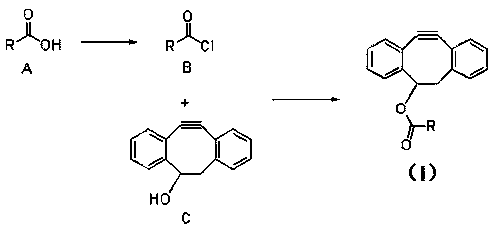
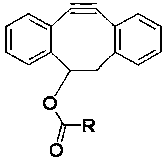
A dibenzocyclooctyne derivative and application thereof
A technology for benzocyclooctynyl alcohol and compound, which is applied in the field of dibenzocyclooctyne derivatives, can solve the problems of tailing, long reaction time, large polarity and the like, achieves easy separation and purification, small steric hindrance, The effect of high ester solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 5,6-Dihydro-11,12-didehydrodibenzo[a,e]cyclooctene-5-yl laurate ( -1) Preparation
[0038] Preparation of Lauroyl Chloride
[0039] Under ice bath at -10°C, dissolve 1 g (4.99 mmol) of lauric acid in 3 ml of freshly distilled thionyl chloride, add dropwise 1 drop of DMF, stir for 20 minutes, raise the temperature to 80°C, react for 3 hours, distill under reduced pressure, evaporate Thionyl chloride and other low-boiling impurities were removed, and the target fraction was collected to obtain 1.07 g of lauroyl chloride as a colorless oily liquid with a content of 99.0% and a yield of 97.8%.
[0040] Preparation of 5,6-dihydro-11,12-didehydrodibenzo[a,e]cyclooctene-5-ester of laurate (I-1)
[0041] Under ice bath at -5°C, dissolve 0.50g (2.27mmol) of 4-dibenzocyclooctynol (compound C) in 15ml of dichloromethane, add dropwise 0.91ml (11.33mmol) of pyridine, stir for 30min, add dropwise Lauroyl chloride 1.0g (4.53mmol), stirred at room temperature for 3h, the reaction s...
Embodiment 2
[0043] Preparation of 5,6-dihydro-11,12-didehydrodibenzo[a,e]cyclooctene-5-ester of myristic acid (I-2)
[0044] Preparation of myristoyl chloride
[0045] Under ice bath at -5°C, dissolve 1g (4.38mmol) of myristic acid in 5ml of freshly steamed thionyl chloride, add 2 drops of DMF dropwise, stir at room temperature for 20 minutes, raise the temperature to 70°C, react for 4h, and distill under reduced pressure , distilled thionyl chloride and other low-boiling impurities, collected the target fraction, and obtained 1.06 g of myristoyl chloride, a colorless oily liquid, with a content of 98.8% and a yield of 98.1%.
[0046] Preparation of 5,6-dihydro-11,12-didehydrodibenzo[a,e]cyclooctene-5-ester of myristic acid (I-2)
[0047]Under ice bath at 0°C, dissolve 0.47g (2.15mmol) of 4-dibenzocyclooctynol (compound C) in 15ml of tetrahydrofuran, add 0.87ml (10.73mmol) of pyridine dropwise, stir for 20min, and add myristoyl chloride dropwise 1.06g (4.29mmol), stirred at room tempera...
Embodiment 3
[0049] Preparation of 5,6-dihydro-11,12-didehydrodibenzo[a,e]cyclooctene-5-ester of palmitate (I-3)
[0050] Preparation of Palmitoyl Chloride
[0051] Under ice bath at 0°C, dissolve 1.0g (3.90mmol) of palmitic acid in 5ml of freshly distilled thionyl chloride, add 2 drops of DMF dropwise, stir at room temperature for 20 minutes, raise the temperature to 80°C, react for 3h, and distill under reduced pressure. Distill thionyl chloride and other low-boiling impurities, collect target fractions, and obtain palmitic acid 5,6-dihydro-11,12-didehydrodibenzo[a,e]cyclooctene-5-ester (I -3) 1.05g, colorless oily liquid, content 99.2%, yield 98.5%.
[0052] Preparation of 5,6-dihydro-11,12-didehydrodibenzo[a,e]cyclooctene-5-ester of palmitate (I-3)
[0053] Under ice bath at 5°C, dissolve 0.40g (1.82mmol) of 4-dibenzocyclooctynol (Compound C) in 15ml of dichloromethane, add 0.73ml (9.1mmol) of triethylamine dropwise, stir for 20min, drop Add 1.0g (3.64mmol) of palmitoyl chloride, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com