Sunscreen molecule, application and sunscreen cream
A technology for sunscreen and sunscreen ingredients, applied in the fields of sunscreen molecules and applications and sunscreens, can solve problems such as skin allergies and human body absorption, and achieve the effects of enhancing light-absorbing ability, strong light-absorbing ability, and accelerating the conversion of light energy into heat energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 (synthesis of sunscreen molecule):
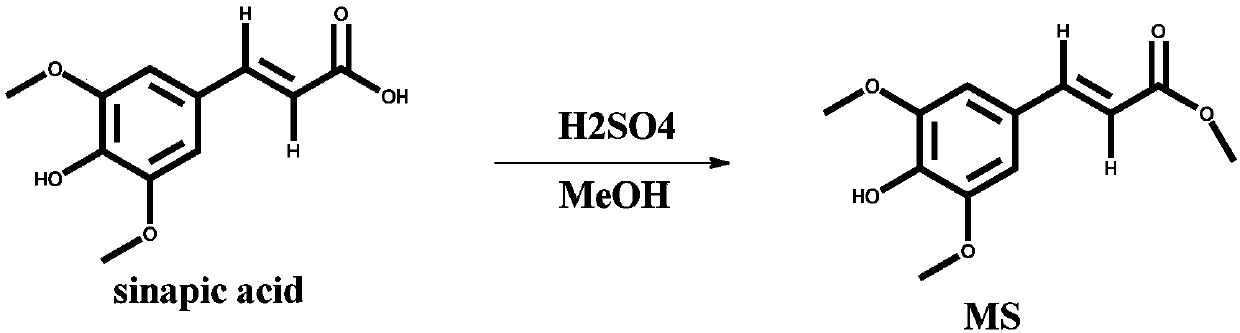
[0022] Such as figure 2 As shown, the structure of the sunscreen molecule adopted in the embodiment is represented by the code MS. In a 100ml single-necked flask, add 500mg sinapic acid, 10ml anhydrous methanol, and two drops of concentrated sulfuric acid with a mass concentration of 98%. The mixture was refluxed at a temperature of 65° for 12 hours. After cooling to room temperature, the reaction mixture was evaporated to dryness with a rotary evaporator, and the residue was dissolved in 50 ml of ethyl acetate, washed 3 times with saturated sodium bicarbonate solution, and then dried over anhydrous sodium sulfate. , and the target product MS was separated by column chromatography. NMR 1 H spectrum and 13 The C spectrum proves that the molecular structure is indeed MS.
Embodiment 2
[0023] Embodiment 2 (preparation of sunscreen cream measures SPF value and compares the sunscreen effect of commercial sunscreen molecules):
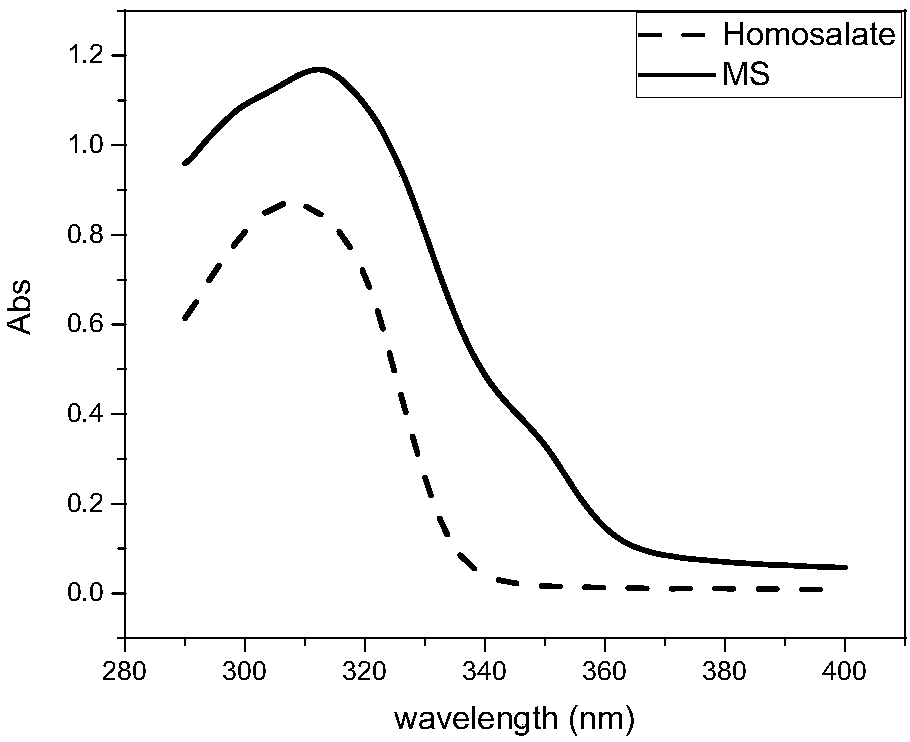
[0024] The synthetic sunscreen molecule MS and homosalate (homosalate) were blended into the commercially available cream, the mass fraction of the molecule was 10%, and stirred with a magnetic stirrer for 24 hours under the condition of avoiding light. After stirring evenly, apply the cream mixture to a quartz glass plate (4*4cm) with a layer of 3M adhesive tape, and the amount of application is 2mg / cm 2 . After half an hour of drying in the dark, use a SHIMADZ UV-2600 ultraviolet-visible absorption spectrometer with a 60mm integrating sphere to measure the absorption spectrum. The absorption spectrum is as follows image 3 As shown, the calculated SPF value of the sunscreen cream containing MS sunscreen molecules is 11, and the SPF value of the sunscreen cream containing homosalate is 3.2.
Embodiment 3
[0025] Embodiment 3 (sunscreen molecule transient absorption experiment):
[0026] The synthesized sunscreen molecule MS was dissolved in methanol solution with a mass fraction of 10%, and its absorbance was measured as 1 with a 1mm thick quartz cuvette. Then femtosecond transient absorption spectroscopy was performed to measure the excited state decay process. The excitation wavelength is 320nm, and the detection wavelength is 400-800nm. The excited state decay curve was measured as Figure 4 shown. After fitting, its excited state lifetime is less than 2ps, that is, the molecule can convert absorbed light energy into heat energy within 2ps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com