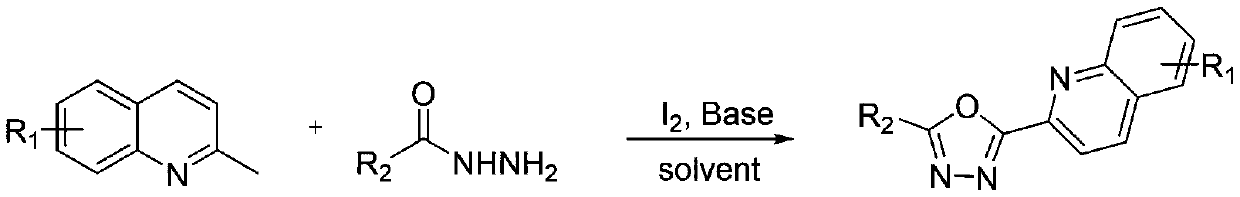
Preparation method of 2-aryl-5-(2-quinolyl)-1,3,4-oxadiazole type compound
A technology for oxadiazoles and compounds, which is applied in the field of preparation of 2-aryl-5--1,3,4-oxadiazoles, can solve complex synthesis methods, harsh reaction conditions, long reaction time, etc. The problem is that the synthesis method is simple, the reaction conditions are simple, and the reaction time is short.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation
[0047] The reaction formula is:
[0048]
[0049] The specific steps are: add 0.6mmol 2-methylquinoline, 0.9mmol elemental iodine, and 2mL dimethyl sulfoxide into a 15mL pressure tube, and stir the reaction at 110°C for 4 hours. After the reaction is cooled, add 0.5mmol Benzohydrazide and 3mmol potassium carbonate were magnetically stirred at 110°C for 6 hours. After the reaction was completed, the reaction solution was extracted, the organic layer was washed, dried, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was petroleum ether / Ethyl acetate = 10:1 (V / V) is the eluent, and the desired product is obtained by column separation and purification. The product is a yellow solid with a yield of 83%.
[0050] The result of the product identification data is: 1 H-NMR (400MHz, CDCl 3 ): δ (ppm) 8.40 (t, J = 8.4 Hz, 2H), 8.33 (s, 1H), 8.27-8.31 (m, 2H), 7.91 (dd, J = 1.2, 8.4 Hz, 1H), 7.67 ( dt, J = 1.2, 8...
Embodiment 2
[0051] Example 2: Preparation
[0052] The reaction formula is:
[0053]
[0054] The specific steps are: add 0.6mmol 2,6-dimethylquinoline, 0.9mmol elemental iodine, and 2mL dimethyl sulfoxide into a 15mL pressure tube, and magnetically stir the reaction at 110°C for 4 hours. After the reaction is cooled, Add 0.5mmol benzoyl hydrazide and 3mmol potassium carbonate, stir magnetically at 110°C for 6 hours. After the reaction is complete, extract the reaction solution, wash the organic layer, dry, and distill under reduced pressure to remove the solvent to obtain a crude product. Petroleum ether / ethyl acetate=10:1 (V / V) is the eluent, and the desired product is obtained by column separation and purification. The product is a yellow solid with a yield of 74%.
[0055] The result of the product identification data is: 1 H-NMR (400MHz, CDCl 3 ): δ(ppm) 8.31(d,J=8.4 Hz,1H),8.24-8.27(m,2H),8.21(d,J=8.4Hz,1H), 8.15(d,J=9.2Hz,1H) ,7.62(d, J=8.0Hz,2H),7.54-7.57(m,2H),7.53-7.54(m,1H),2.55(s,...
Embodiment 3
[0056] Example 3: Preparation
[0057] The reaction formula is:
[0058]
[0059] The specific steps are: add 0.6mmol 6-fluoro-2-methylquinoline, 0.9mmol elemental iodine, and 2mL dimethyl sulfoxide into a 15mL pressure tube, and magnetically stir the reaction for 4 hours at 110°C. After the reaction is cooled , Add 0.5mmol benzoyl hydrazide and 3mmol potassium carbonate, stir magnetically at 110°C for 6 hours. After the reaction is complete, extract the reaction solution, wash the organic layer, dry, and distill under reduced pressure to remove the solvent to obtain the crude product. Ethanol / dichloromethane was recrystallized and the product was a yellow solid with a yield of 70%.
[0060] The result of the product identification data is: 1 H-NMR (400MHz, CDCl 3 ): δ (ppm) 8.40 (d, J = 8.4 Hz, 1H), 8.30 (s, 1H), 8.29 (d, J = 8.0 Hz, 1H), 8.27 (t, J = 2.4 Hz, 1H), 8.25 (d,J=2.4Hz,1H),7.57-7.59(m,1H),7.57(t,J=2.4Hz,2H),7.55(d,J=1.6Hz,1H), 7.51(dd,J= 3.2,.8Hz,1H), 13 C-NMR (100MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com